138584
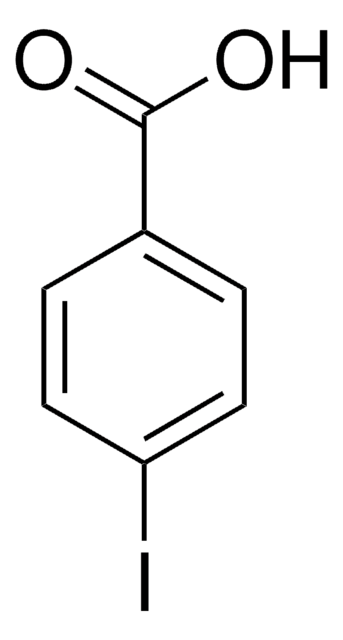
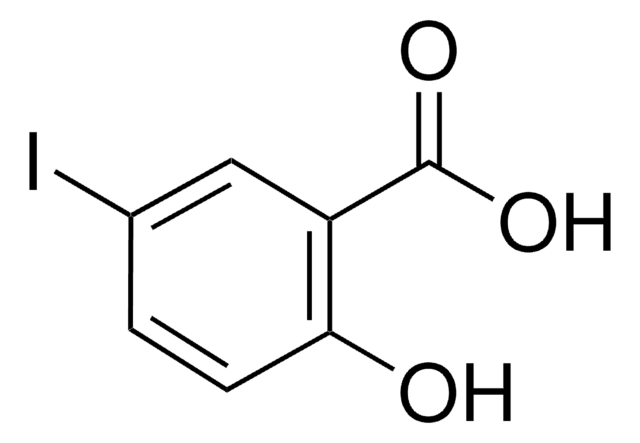
3-Iod-benzoesäure
98%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
IC6H4CO2H
CAS-Nummer:
Molekulargewicht:
248.02
Beilstein:
971088
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
mp (Schmelzpunkt)
185-187 °C (lit.)
Funktionelle Gruppe
carboxylic acid
iodo
SMILES String
OC(=O)c1cccc(I)c1
InChI
1S/C7H5IO2/c8-6-3-1-2-5(4-6)7(9)10/h1-4H,(H,9,10)
InChIKey
KVBWBCRPWVKFQT-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
3-Iodobenzoic acid is added as UV absorbing background electrolyte in separation of uncharged cyclodextrins and their derivatives by capillary electrophoresis.
Anwendung
3-Iodobenzoic acid was used in solid phase synthesis of γ-turn mimetic library.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
A γ-Turn Mimetic Library: Development and Production.
Kocis P, et al.
High-Throughput Synthesis: Principles and Practices, 65-65 (2010)
G Vaidyanathan et al.
Bioconjugate chemistry, 1(6), 387-393 (1990-11-01)
We have previously shown that use of N-succinimidyl 3-iodobenzoate (SIB) for radioiodination of monoclonal antibodies (MAbs) decreases the loss of radioiodine in vivo compared to MAbs labeled by using conventional methods. Herein, the synthesis of N-succinimidyl 2,4-dimethoxy-3-(trialkylstannyl)benzoates (alkyl = Me
M Pumera et al.
Fresenius' journal of analytical chemistry, 369(7-8), 666-669 (2001-05-24)
A fast and simple capillary electrophoretic method suitable for the determination of native alpha-, beta-, gamma-cyclodextrins, their randomly substituted tert-butyl derivatives (average degree of substitution 3.8-4.4), heptakis (2,6-di-O-methyl)- and heptakis (2,3,6-tri-O-methyl)-beta-cyclodextrin was developed. Naphthyl-2-sulfonic acid (2-NSA), 3-iodobenzoic acid (3-IBA) and
Negative inotropic effects of Na-salicylate and three congeners on the guinea-pig Langendorff heart.
H Brasch
Archives internationales de pharmacodynamie et de therapie, 262(2), 242-249 (1983-04-01)
In guinea-pig Langendorff hearts, Na-salicylate (1.9, 3.8 and 7.6 mmol/l) concentration-dependently reduced the contractile force (--9.1, --51.0 and --75.1%, respectively) and the coronary resistance. The influence of the uncoupling agent 2.4-dinitrophenol (0.02 mmol/l) was comparable to that of the largest
Pablo Wessig et al.
Molecules (Basel, Switzerland), 18(1), 1314-1324 (2013-01-23)
Various 1,6- and 1,8-naphthalenophanes were synthesized by using the Photo-Dehydro-Diels-Alder (PDDA) reaction of bis-ynones. These compounds are easily accessible from ω-(3-iodophenyl)carboxylic acids in three steps. The obtained naphthalenophanes are axially chiral and the activation barrier for the atropisomerization could be
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.