Silicides: Promising Thermoelectrics
Mikhail I., Fedorov, Vladimir K., Zaitsev
Ioffe Physical-technical Institute, St. Petersburg, Russia
Material Matters™, 2022, 6.4 Material Matters™ Publications
Introduction
In recent years, the price of tellurium, a key component in the best-performing thermoelectric materials, has increased significantly. This raises the question, "Is it economically viable to produce thermoelectric generators on an industrial scale?". The main thermoelectric generator material is bismuth telluride, which has a high enough dimensionless figure of merit (ZT ~ 1) at room temperature to be practical for energy generating applications. The dimensionless figure of merit is described as:
ZT = S2σT/κ
where S is the Seebeck coefficient, σ and κ are the electrical and thermal conductivities, respectively, and T is the absolute temperature. The other intermetallic materials used for higher operating temperatures are PbTe for n-type and GeTe for p-type semiconductors. Both these materials are expensive, especially GeTe. Due to increasing tellurium costs, it is necessary to find alternative materials that have comparable figures of merit.
Silicides represent a group of promising thermoelectric materials for several reasons. Firstly, silicon is the fourth most abundant element and among the most widespread elements of the Earth′s crust. Additionally, silicides do not contain toxic elements, are ecologically benign, and represent various mechanisms of electron transport. The primary characteristics of silicides are presented in Table 1, which show the latest results obtained in the field of thermoelectrics.1,2
INCREASING THE THERMOELECTRIC FIGURE OF MERIT
As shown in the previous equation, the thermoelectric figure of merit is calculated by multiplying the square of the Seebeck coefficient by the electrical conductivity, dividing by the thermal conductivity, and multiplying by the absolute temperature. The Seebeck coefficient and electrical conductivity both depend on charge carrier density and cannot be simultaneously increased in a practical manner. There is an optimum charge carrier density that delivers the highest nominator (power factor) of the thermoelectric figure of merit.
Only one parameter can be changed independently of charge carrier density; this is the thermal conductivity, which consists of two parts — phonon and electron. These parts can be altered more or less independently. The method of creating solid solutions allows significant reductions in thermal conductivity. In this method, the solution of two isovalent materials with the same crystal structure could be used to increase phonon scattering, thereby reducing thermal conductivity. Maximizing ZT requires minimal thermal conductivity.
Another method of increasing the figure of merit is to increase the density of states (DOS). This is very difficult to achieve in some materials, because the density of states is typically dependent on the band structure of a material, and is therefore immutable. However, in solid solutions, it is sometimes possible to alter the band structure to increase the figure of merit.
Two Types of Thermoelectric Generators
There are two main types of thermoelectric generators, schematics diagrams for which are shown in Figure 1.
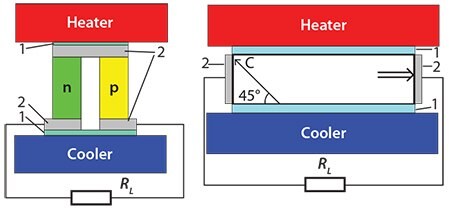
Figure 1. Left) Traditional thermoelement with n- and p-type legs. Right) Thermoelement with an anisotropic material.
The left scheme shows a traditional thermoelement, consisting of two legs with different types of conductivity (n and p). The right scheme shows another type of thermoelement, which includes a material with an anisotropic Seebeck coefficient. Here, the voltage of the thermoelement is determined by the difference of longitudinal and transverse Seebeck coefficients. The thermoelectric figure of merit for this case (ZTa) may be written as:
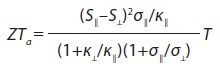
where symbols || (along the axis of higher symmetry) and ⊥ (across the same axis) denote the direction used to measure this property. In this type of thermoelement, voltage is shown as:
V=1/2(S||-S⊥)×ΔT×l/d
where V - is the voltage of the thermoelement, ΔT is the temperature differential, l - is the length between contacts, and d - is the thickness of the element. Taking into account that ΔT is closely related to the thickness of the element, it is possible to use the following expression for volt/watt sensitivity:
V/Q=1/2(S||-S⊥)/h/κ45
where Q is the heat flux through the thermoelement, κ45 is the average thermal conductivity at an angle of 45° to the axis of higher symmetry, and h is the width of the strip of thermoelectric material. This formula is valid when contact resistance is much lower than the resistance of the strip. The main advantage of this kind of thermoelement is the absence of contacts in the hot junctions.
Synthesis of Silicides
As discussed previously, silicides are a promising group of nontoxic and cost-efficient materials for thermoelectrics. These materials can be prepared by a variety of methods, particularly those that allow for property measurements. For example, chromium disilicide, higher manganese silicide, ruthenium sesquisilicide, and cobalt monosilicide are prepared by either the Bridgman or Czochralski methods of single crystal preparation. The Czochralski method has also been used for the preparation of rhenium silicide. The floating zone method is also used to synthesize many silicides, especially for refractory types. In laboratory studies of the physical properties of thermoelements, magnesium silicide-based materials are prepared by direct melting of components with subsequent annealing steps.
Transition Metal Silicides
The first five silicides in Table 1 are referred to as “higher silicides” because they have the maximum silicon content within the corresponding system. With the exception of CrSi2, which crystallizes in a hexagonal structure, all other silicides have crystal structures that are either tetragonal or a slight variant of a tetragonal structure. All these silicides have high anisotropies of the Seebeck coefficient, making them viable candidates for use in anisotropic thermoelectric generators. As these materials have very large temperature intervals of this anisotropy, they can be used in sensors with high dynamic ranges. Interestingly, in ReSi1.75, the anisotropy of the Seebeck coefficient is very large; in one sample, the coefficient was positive, while in another sample, it was negative when measured along the highest symmetry axis. These types of applications require each sample to include two Seebeck coefficients with opposite signs, operating in different directions.
The last two silicides in Table 1 crystallize in a cubic structure. CoSi is almost a semimetal, which allows its use as a contact layer in thermoelectric generators. Cobalt monosilicide has one of the highest power factors of all thermoelectric materials (N = S2σ) but a lower overall thermoelectric figure of merit (Figure 2 and Figure 3). Two other silicide materials also have very high power factors. The first is a solid solution of Mg2Si (Prod. No. 343196) and Mg2Sn, which has N ~ 45 W/(mK2) at 600 K; the other is CrSi2, produced by the synthesis of micron scale powders. In the case of CrSi2, there is some anisotropy of power factor.

Figure 2.Power factor of some silicides.
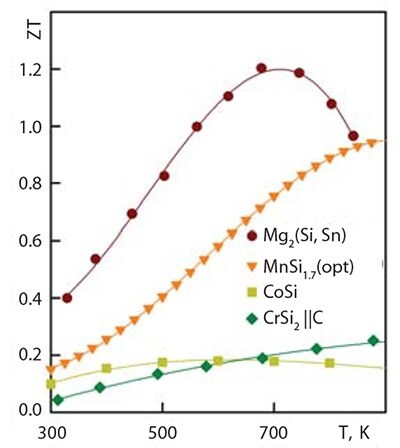
Figure 3.Thermoelectric figure of merit of some silicides.
Interest in the use of chromium disilicide started following an attempt to produce the silicide by high-temperature flux growth in molten tin.3 Needles formed by this method retained tin within their structure. After etching the tin, the final product contained channels with internal diameters of approximately 100 μm (Figure 4). The structural anisotropy is reflected within the varying properties between parallel and perpendicular directions.
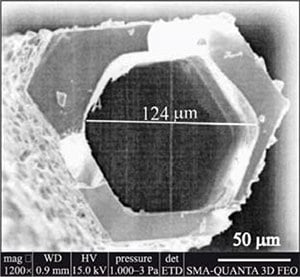
Figure 4. SEM micrograph of CrSi2 tubes obtained from flux growth in molten tin illustrating the hollow interior.
The most efficient thermoelectric material among all transition metal silicides is higher manganese silicide. It has a figure of merit significantly lower than that of Mg2Si-based materials, but is still in the region of ZT ~ 0.9 at 870 K. The best material must be doped in a complex manner to produce the highest value for figure of merit. The material must contain at least three dopants: one to reduce thermal conductivity, one to optimize the crystalline lattice, and another to optimize the charge carrier density. In an idealized response, the material will have the characteristics shown in Figure 3 for Mg2(Si,Sn). In comparison with Mg2Si-based materials, higher manganese silicide has greater mechanical and chemical strength, which allows its use in air and aggressive media with a limited amount of protection.
SOLID SOLUTIONS BASED ON MG2SI
The other silicon-based materials are the solid solutions of Mg2Si and Mg2Sn.10 These materials have high thermal conductivities (κ, in W/(m⋅K); 8.36 Mg2Si and 6.97 Mg2Sn) but, in solid solutions, even 10% of the other component reduces thermal conductivity by a significant margin. The conduction band of these compounds consists of two sub-bands located in the X-point of the Brillouin zone. The lower band of Mg2Si consists of the states of Si, whereas the lower band of Mg2Sn consists of the Mg states. The change of position of the sub-bands allows for an increased density of states in the conduction band. There are therefore two mechanisms to increase the figure of merit in solid solutions based on Mg2Si and Mg2Sn. It is possible to reduce the thermal conductivity and to find the optimum position of the sub-bands within the region of lower thermal conductivity.
There are two optimum impurities which allow control of the charge carrier density — Bi and Sb. Both impurities work well to optimize the density, but the mechanical properties of material doped with Bi are slightly better. As shown in Figure 5, the results of the present study are very similar to those previously reported by Isoda et al.12,13 and Zhang et al.14

Figure 5. Figures of merit for solid solutions of Mg2Si and Mg2Sn. 1) Mg2Si0.4Sn0.6 <Sb>;10 2) Mg2Si0.4Sn0.6 <Bi>;10 3) Mg2Si0.36Ge0.04Sn0.6;10 4) Mg2Si0.5Sn0.5;12 5) Mg2Si0.5Sn0.5;13 6)Mg2Si0.4Sn0.6.14
In this system, it is difficult to produce a p-type material with a ZT > 0.5,4 due to the position of the valence band in the Γ-point of the Brillouin zone and a relatively low energy gap in Mg2Sn.
Conclusions
Thermoelectrical research could benefit greatly from a single rule to determine the most appropriate material for a given application. Unfortunately, as no such method exists, all cases require examination of the interactions between many parameters. Therefore it is not always possible to determine the optimal material constituents.
As shown above, the higher transition metal silicides are very well-suited for anisotropic thermoelectric conversion. Essential anisotropy of the Seebeck coefficient, together with good mechanical properties, allows production of reliable anisotropic thermoelectric converters.
For traditional thermoelectric modules, the situation is different. There is no coupling of the materials that have high ZT values for both signs. In this case, it is necessary to produce a thermoelectric generator comprising significantly different materials that may result in the device having low reliability.
References
To continue reading please sign in or create an account.
Don't Have An Account?