All Photos(1)
About This Item
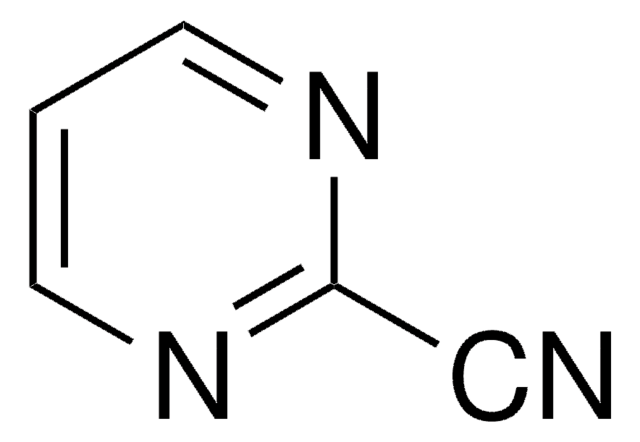
Empirical Formula (Hill Notation):
C11H11FN2O2
CAS Number:
Molecular Weight:
222.22
EC Number:
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
Recommended Products
form
crystalline
mp
280-285 °C (dec.) (lit.)
storage temp.
−20°C
SMILES string
NC(Cc1c[nH]c2cc(F)ccc12)C(O)=O
InChI
1S/C11H11FN2O2/c12-7-1-2-8-6(3-9(13)11(15)16)5-14-10(8)4-7/h1-2,4-5,9,14H,3,13H2,(H,15,16)
InChI key
YMEXGEAJNZRQEH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
6-Fluoro-DL-tryptophan (6-F-TRP), a serotonin (5-HT) synthesis inhibitor, is metabolized in the brain and may be useful for tracing neuronal serotoninergic pools. 6-F-TRP is used as a competitive inhibitor of tryptophan binding to albumin and passage through the blood brain barrier (BBB).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S Miwa et al.
Journal of neurochemistry, 48(5), 1577-1580 (1987-05-01)
After intraperitoneal injection of rats with 6-fluorotryptophan (6-FT), brain 5-hydroxytryptamine (5-HT) levels decreased exponentially over 1 h. Depletion was dose-dependent and maximum depletion was observed at 200 mg/kg. 6-FT (200 mg/kg) did not significantly alter the content of 5-hydroxyindoleacetic acid.
E Chanut et al.
Journal of neural transmission. General section, 96(2), 105-112 (1994-01-01)
6-Fluoro-serotonin (6F-5-HT) was previously identified in the rat brain after peripheral administration of 6-fluoro-DL-tryptophan, a serotonin (5-HT) synthesis inhibitor. These present studies, performed with rat brain synaptosomes show that: i-neuronal 6F-5-HT uptake partly involved the 5-HT transporter since it was
E Li et al.
The Journal of biological chemistry, 264(29), 17041-17048 (1989-10-15)
Rat cellular retinol-binding protein II (CRBP II) is a 15.6-kDa intestinal protein which binds all-trans-retinol and all-trans-retinal but not all-trans-retinoic acid. We have previously analyzed the interaction of Escherichia coli-derived rat apoCRBP II with several retinoids using fluorescence spectroscopic techniques.
A H Baugher et al.
Biophysical journal, 75(5), 2574-2576 (1998-10-28)
Rotational-echo double-resonance (REDOR) 13C NMR spectra (with 19F dephasing) have been obtained of 6-fluorotryptophan complexed by a polymeric amphiphilic nanosphere consisting of a polystyrene core covalently attached to a poly(acrylic acid)-polyacrylamide shell. The REDOR spectra show that aromatic carbons from
Induction of anthranilate synthase activity by elicitors in oats.
Matsukawa T, Ishihara A, Iwamura H.
Zeitschrift fur Naturforschung, 57, 121-128 (2002)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service