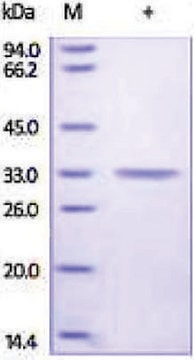
This product is a mixture of the CA-I and CA-I isozymes. The ratio has not been determined.
C3934
Carbonic Anhydrase from bovine erythrocytes
lyophilized powder, ≥2,000 W-A units/mg protein
Synonym(s):
Carbonate Dehydratase, Carbonate Hydrolyase
About This Item
Recommended Products
biological source
bovine erythrocytes
Quality Level
form
lyophilized powder
specific activity
≥2,000 W-A units/mg protein
mol wt
30 kDa
application(s)
diagnostic assay manufacturing
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
Unit Definition
inhibitor
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Objective: To standardize a procedure for the enzymatic assay of Carbonic Anhydrase (EC 4.2.1.1) for Wilbur-Anderson Units.
-
Dear who is concern, I would like to know which isoform of carbonic anhydrase this is.
1 answer-
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
What is the molecular weight of Product C3934, Carbonate Dehydratase?
1 answer-
Because there are a number of closely related isoforms, the molecular weight typically used is approximately 30 kDa. This product is expected to be a mixture of isoforms.
Helpful?
-
-
Can stock solutions of Product C3934, Carbonate Dehydratase, be made and stored?
1 answer-
Carbonic anhydrase solutions can be prepared in water or buffer at concentrations up to 10 mg/mL. It is common for there to be some particulates or haziness in the solution. No data on solution stability has been collected by Sigma-Aldrich, so we recommend solutions be prepared fresh for use.
Helpful?
-
-
What is the purity of Product C3934, Carbonate Dehydratase?
1 answer-
Since C3934 is a mixture of isoforms, no purity claim is made. The product is packaged and offered based on its enzymatic activity.
Helpful?
-
Active Filters
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service