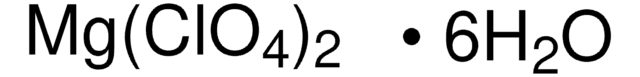About This Item
Recommended Products
grade
ACS reagent
form
flakes
powder, chunks or granules
reaction suitability
reagent type: oxidant
concentration
>10% Mg (EDTA titration)
impurities
≤0.005 meq/g Titr. free acid
≤0.025 meq/g Titr. base
loss
≤8% loss on drying
suitability
passes test for moisture absorption
SMILES string
[Mg++].[O-]Cl(=O)(=O)=O.[O-]Cl(=O)(=O)=O
InChI
1S/2ClHO4.Mg/c2*2-1(3,4)5;/h2*(H,2,3,4,5);/q;;+2/p-2
InChI key
MPCRDALPQLDDFX-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Preparation of α-aminophosphonates.
- Enantioselective Diels-Alder reaction between cyclopentadiene and 3-acryloyl-1,3-oxazolin-2-one.
- Preparation of imines and phenylhydrazones.
- Protection of alcohols in the form of t-butyl ethers.
- α-Aminophosphonates via three-component reaction between an amine, an aldehyde or a ketone and a di-/trialkyl phosphite.
- Imines and phenylhydrazones by the condensation of carbonyl compounds with amines and phenylhydrazine.
- Knoevenagel adducts via Knoevenagel condensation between β-diketones and aliphatic or aromatic aldehydes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service