341220
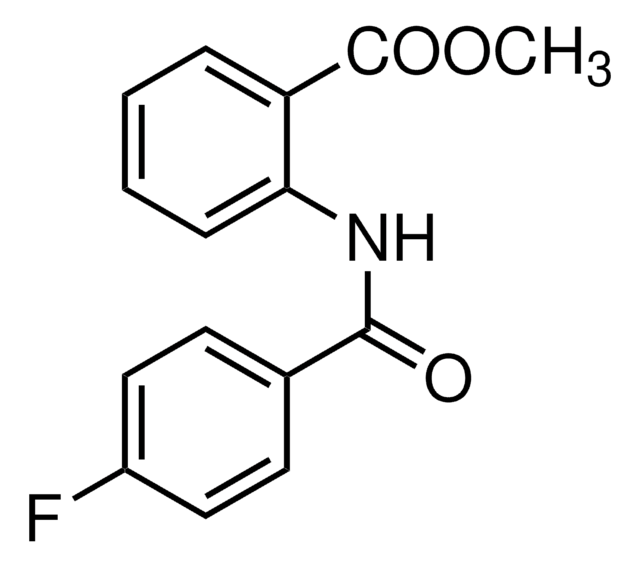
Exo1
A cell-permeable methylanthranilate analog that reversibly inhibits vesicular traffic from ER to Golgi in mammalian cells by inducing tubulation and collapsing of the Golgi membrane.
Synonym(s):
Exo1, 2-(4-Fluorobenzoylamino)methylbenzoate, 2-(4-Fluorobenzoylamino)benzoic Acid Methyl Ester, ER Export Inhibitor I
About This Item
Recommended Products
Quality Level
Assay
≥95% (HPLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
protect from light
color
white
solubility
DMF: 25 mg/mL
DMSO: 25 mg/mL
ethanol: 5 mg/mL
methanol: 5 mg/mL
shipped in
ambient
storage temp.
2-8°C
InChI
1S/C15H12FNO3/c1-20-15(19)12-4-2-3-5-13(12)17-14(18)10-6-8-11(16)9-7-10/h2-9H,1H3,(H,17,18)
InChI key
KIAPWMKFHIKQOZ-UHFFFAOYSA-N
General description
Biochem/physiol Actions
Golgi ARF1 GTPase
Packaging
Warning
Reconstitution
Other Notes
Legal Information
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service