668540
(2S,5S)-(–)-5-Benzyl-3-methyl-2-(5-methyl-2-furyl)-4-imidazolidinone
95%
Synonym(s):
(2S,5S)-3-Methyl-2-(5-methyl-2-furanyl)-5-(phenylmethyl)-4-imidazolidinone
About This Item
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.5598
SMILES string
CN1[C@H](N[C@@H](Cc2ccccc2)C1=O)c3ccc(C)o3
InChI
1S/C16H18N2O2/c1-11-8-9-14(20-11)15-17-13(16(19)18(15)2)10-12-6-4-3-5-7-12/h3-9,13,15,17H,10H2,1-2H3/t13-,15-/m0/s1
InChI key
IQIMPHPFMHVWBZ-ZFWWWQNUSA-N
Related Categories
Application
- Enantioselective organocatalytic transfer hydrogenation of cycloalkenones with tert-Bu Hantzsch ester as source of hydrogen
- Alkylation of indoles with an α,β-disubstituted α,β-unsaturated aldehyde in the enantioselective preparation of a selective serotonin reuptake inhibitor
- MacMillan reaction
Legal Information
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
>230.0 °F
Flash Point(C)
> 110 °C
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Discover Professor David MacMillan's groundbreaking metal-free asymmetric catalysis using imidazolidinone-based organocatalysts for versatile transformations.
Discover Professor David MacMillan's groundbreaking metal-free asymmetric catalysis using imidazolidinone-based organocatalysts for versatile transformations.
Discover Professor David MacMillan's groundbreaking metal-free asymmetric catalysis using imidazolidinone-based organocatalysts for versatile transformations.
Discover Professor David MacMillan's groundbreaking metal-free asymmetric catalysis using imidazolidinone-based organocatalysts for versatile transformations.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

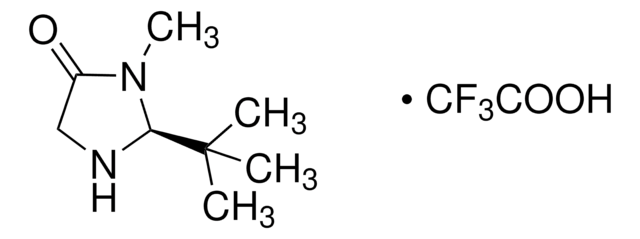
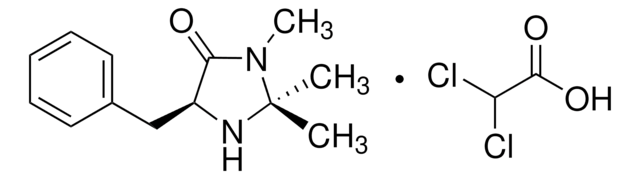
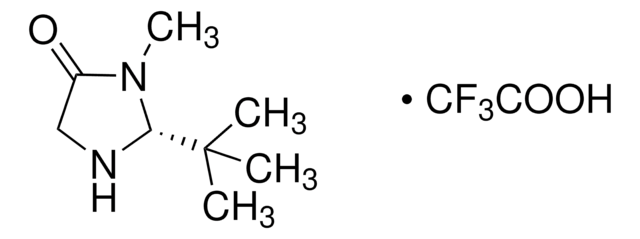
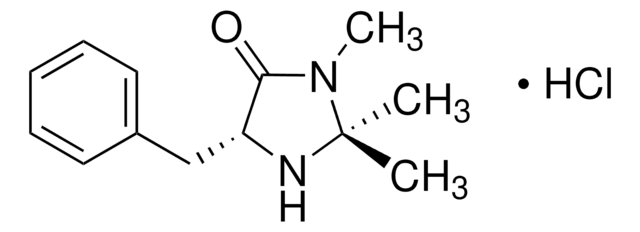
![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol trimethylsilyl ether 97%](/deepweb/assets/sigmaaldrich/product/structures/396/398/09a397b1-b5f5-420f-98da-adf9017cef56/640/09a397b1-b5f5-420f-98da-adf9017cef56.png)



