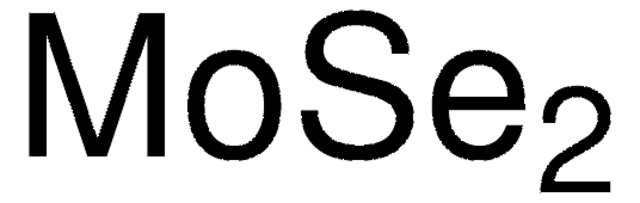367168
Tin(II) sulfide
96%
Synonym(s):
Herzenbergite, Stannous sulfide, Tin monosulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
SSn
CAS Number:
Molecular Weight:
150.78
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.23
Assay:
96%
Recommended Products
Assay
96%
density
5.22 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
S=[SnH2]
InChI
1S/S.Sn
InChI key
AFNRRBXCCXDRPS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C Mundus et al.
Solid state nuclear magnetic resonance, 7(2), 141-146 (1996-11-01)
Non-spinning and magic angle spinning (MAS) 119Sn nuclear magnetic resonance (NMR) spectra of the binary tin sulphides SnS and SnS2 as well as of a number of stoichiometric compounds from the ternary system Na2S-SnS2 have been recorded. The isotropic chemical
Yaping Du et al.
Nanoscale, 5(4), 1456-1459 (2013-01-12)
A facile, environmentally friendly, and economical synthetic route for production of large-amounts (gram scale) of two-dimensional (2D) layered SnS(2) nanoplates is presented. The electrode fabricated from the SnS(2) nanoplate exhibits excellent lithium-ion battery performance with highly reversible capacity, good cycling
Hiroki Tsukigase et al.
Journal of nanoscience and nanotechnology, 11(3), 1914-1922 (2011-04-01)
SnS-sensitized TiO2 electrodes were applied in quantum dot-sensitized solar cells (QDSSCs) which are environmentally more favorable than conventional Cd or Pb-chalcogenide-sensitized electrodes. SnS nanoparticles were well-distributed over the surface of TiO2 nanoparticles by the successive ionic layer adsorption and reaction
Tetrahedral zinc blende tin sulfide nano- and microcrystals.
Eric C Greyson et al.
Small (Weinheim an der Bergstrasse, Germany), 2(3), 368-371 (2006-12-29)
Hyunju Chang et al.
The journal of physical chemistry. B, 109(1), 30-32 (2006-07-21)
First principles calculations are used to predict the stability and electronic structures of SnS(2) nanotubes. Optimization of several structures and their corresponding strain energies confirm the stability of SnS(2) nanotube structures. Band structure calculations show that SnS(2) nanotubes could have
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service