All Photos(1)
About This Item
Empirical Formula (Hill Notation):
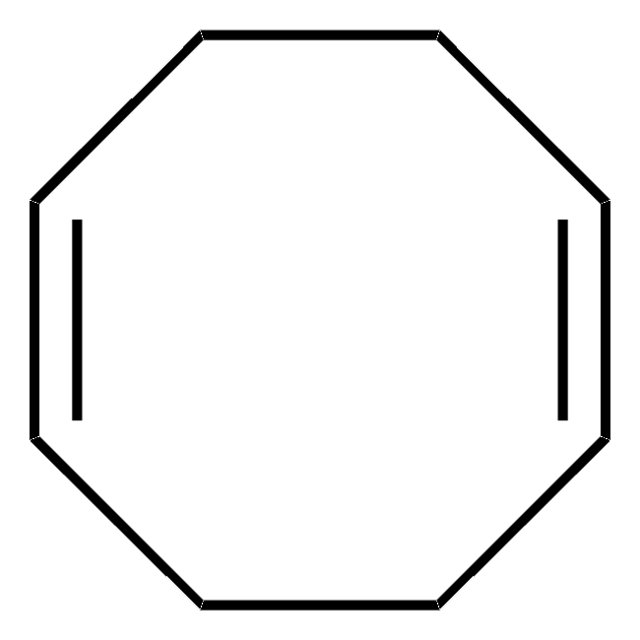
C8H12
CAS Number:
Molecular Weight:
108.18
Beilstein:
2321915
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
Assay
≥95% (GC)
refractive index
n20/D 1.494
bp
142-144 °C (lit.)
density
0.873 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
C1CCC=CC=CC1
InChI
1S/C8H12/c1-2-4-6-8-7-5-3-1/h1-4H,5-8H2/b3-1-,4-2-
InChI key
RRKODOZNUZCUBN-CCAGOZQPSA-N
Related Categories
General description
Enantiodifferentiating geometrical photoisomerizations of (Z,Z)-1,3-cyclooctadiene was studied using "cyclodextrin nanosponge", as a supramolecular sensitizing host. Dendrimer-encapsulated Pd-Rh bimetallic nanoparticle catalyzed partial hydrogenation of 1,3-cyclooctadiene has been reported.
Application
1,3-Cyclooctadiene has been used in the preparation of 2-cyclooct-2-en-1-yl-1,3-cyclooctadiene, a novel bicyclic triene.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
77.0 °F - closed cup
Flash Point(C)
25 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Metal-mediated dimerization of 1, 3-cyclooctadiene to 2-cyclooct-2-en-1-yl-1, 3-cyclooctadiene: a novel bicyclic triene.
Debad JD, et al.
Journal of the American Chemical Society, 115(5), 2051-2052 (1993)
Wenting Liang et al.
Beilstein journal of organic chemistry, 8, 1305-1311 (2012-09-29)
Enantiodifferentiating geometrical photoisomerizations of (Z)-cyclooctene and (Z,Z)-1,3-cyclooctadiene were performed by using the pyromellitate-linked cyclodextrin network polymer, termed "cyclodextrin nanosponge (CDNS)", as a supramolecular sensitizing host. The photochirogenic behavior of the nanosponges incorporating β- or γ-cyclodextrin was significantly different from that
Partial hydrogenation of 1, 3-cyclooctadiene using dendrimer-encapsulated Pd-Rh bimetallic nanoparticles.
Chung Y-M and Rhee H-K.
J. Mol. Catal. A: Chem., 206(1), 291-298 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![Bicyclo[2.2.1]hepta-2,5-diene 98%](/deepweb/assets/sigmaaldrich/product/structures/304/819/dfa7c176-c370-4fb5-acf1-28d751241a50/640/dfa7c176-c370-4fb5-acf1-28d751241a50.png)