All Photos(1)
About This Item
Linear Formula:
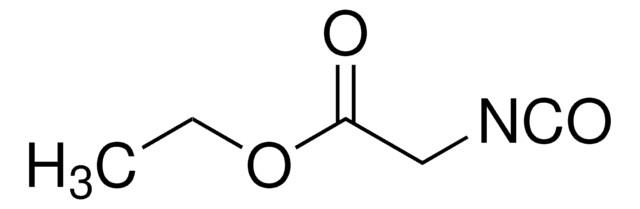
SCNCH2CO2C2H5
CAS Number:
Molecular Weight:
145.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.502 (lit.)
bp
104-106 °C/7 mmHg (lit.)
density
1.171 g/mL at 25 °C (lit.)
functional group
amine
ester
isothiocyanate
storage temp.
2-8°C
SMILES string
CCOC(=O)CN=C=S
InChI
1S/C5H7NO2S/c1-2-8-5(7)3-6-4-9/h2-3H2,1H3
InChI key
IYPSSPPKMLXXRN-UHFFFAOYSA-N
Application
Ethyl isothiocyanatoacetate has been used in the synthesis of:
- fused pyrimidines
- ethyl (3-substituted 5-thioxo-1,2,4-triazolin-4-yl)acetates via addition-cyclization reaction with carboxylic acid hydrazides in the presence of sodium ethoxide
- 3-O-amino-2-thiohydantoins
- quinazoline, benzothienopyrimidine and benzofuropyrimidine
- thiazolo[5,4-d]thiazole
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
228.2 °F - closed cup
Flash Point(C)
109 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Z Chowdhury et al.
Chemical & pharmaceutical bulletin, 49(4), 391-395 (2001-04-20)
o-Aminonitrile or o-aminoester compounds were cyclized to fused pyrimidines by reacting with ethyl iso(thio)cyanatoacetate in pyridine, and then were methylated, halogenated and subsequently displaced by the amines studied.
Synthesis of 3-O-amino-2-thiohydantoins.
Ryczek J.
Journal of Heterocyclic Chemistry, 40(4), 665-670 (2003)
Addition-cyclization reactions of ethyl isothiocyanatoacetate with carboxylic acid hydrazides.
Veverka M and Marchalin M.
Collection of Czechoslovak Chemical Communications, 52(1), 113-119 (1987)
Improved access to thiazolo [5, 4-d] thiazole and thieno [2, 3-d] thiazole.
Rossler A and Boldt P.
Journal of the Chemical Society. Perkin Transactions 1, 4, 685-688 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service