255866
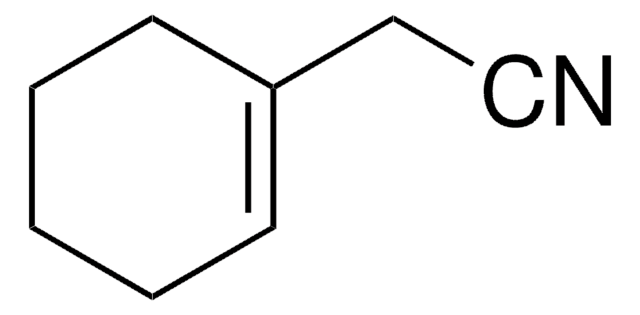
2-(1-Cyclohexenyl)ethylamine
97%
Synonym(s):
1-Cyclohexene-1-ethanamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H9CH2CH2NH2
CAS Number:
Molecular Weight:
125.21
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.4865 (lit.)
bp
53-54 °C/2.5 mmHg (lit.)
density
0.898 g/mL at 25 °C (lit.)
SMILES string
NCCC1=CCCCC1
InChI
1S/C8H15N/c9-7-6-8-4-2-1-3-5-8/h4H,1-3,5-7,9H2
InChI key
IUDMXOOVKMKODN-UHFFFAOYSA-N
Application
2-(1-Cyclohexenyl)ethylamine has been employed:
- as substrate for allylic hydroxylation reaction
- in preparation of thin films and single crystals of 2-(1-cyclohexenyl)ethyl ammonium lead iodide, used to fabricate optoelectronic-compatible heterostructures
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
136.4 °F - closed cup
Flash Point(C)
58 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K Pradeesh et al.
Optics express, 17(24), 22171-22178 (2009-12-10)
Optoelectronic-compatible heterostructures are fabricated from layered inorganic-organic multiple quantum wells (IO-MQW) of Cyclohexenyl ethyl ammonium lead iodide, (C(6)H(9)C(2)H(4)NH(3))(2)PbI(4) (CHPI). These hybrids possess strongly-resonant optical features, are thermally stable and compatible with hybrid photonics assembly. Room-temperature strong-coupling is observed when these
Facile stereoselective allylic hydroxylation by dopamine. beta.-monooxygenase.
Sirimanne SR and May SW.
Journal of the American Chemical Society, 110(22), 7560-7561 (1988)
S R Sirimanne et al.
The Biochemical journal, 306 ( Pt 1), 77-85 (1995-02-15)
The reaction of dopamine beta-monooxygenase (DBM; EC 1.14.17.1) with the prototypical non-conjugated olefinic substrate, 2-(1-cyclohexenyl)ethylamine (CyHEA) [see Sirimanne and May (1988) J. Am. Chem. Soc. 110, 7560-7561], was characterized. CyHEA undergoes facile DBM-catalysed allylic hydroxylation to form (R)-2-amino-1-(1-cyclohexenyl)ethanol (CyHEA-OH) without
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service