All Photos(2)
About This Item
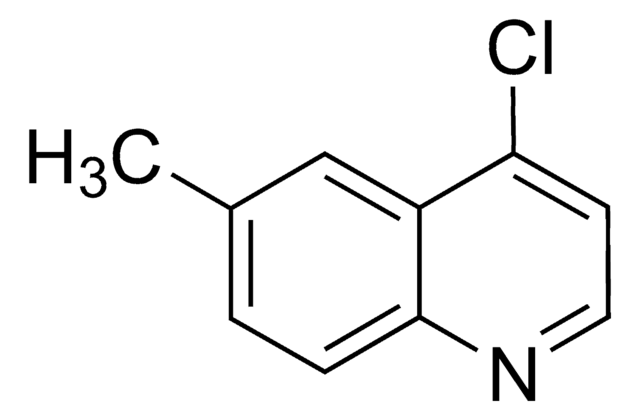
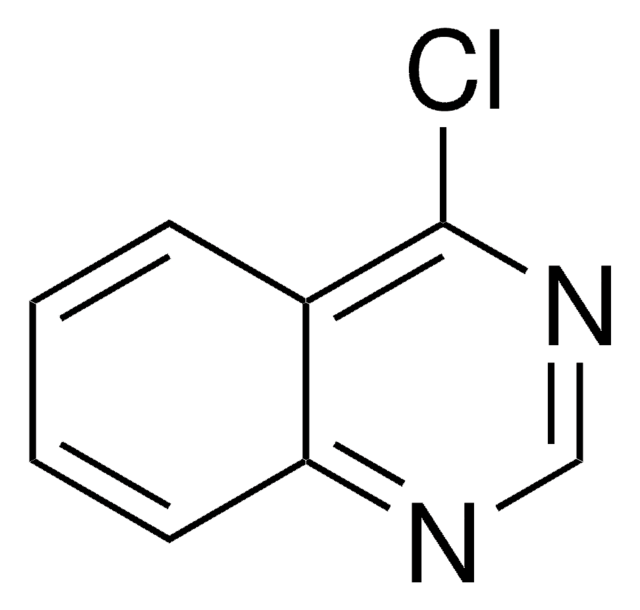
Empirical Formula (Hill Notation):
C10H5ClF3N
CAS Number:
Molecular Weight:
231.60
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
69-71 °C (lit.)
solubility
chloroform: soluble 25 mg/mL, clear, colorless
SMILES string
FC(F)(F)c1ccc2c(Cl)ccnc2c1
InChI
1S/C10H5ClF3N/c11-8-3-4-15-9-5-6(10(12,13)14)1-2-7(8)9/h1-5H
InChI key
LLRQVSZVVAKRJA-UHFFFAOYSA-N
Application
4-Chloro-7-(trifluoromethyl)quinolone was used in the synthesis of (4-substituted phenyl-1-piperazinyl) alkyl 2-aminobenzoates and 2-aminonicotinates.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
P M Manoury et al.
Journal of medicinal chemistry, 22(5), 554-559 (1979-05-01)
A series of (4-substituted phenyl-1-piperazinyl)alkyl 2-aminobenzoates and 2-aminonicotinates has been prepared and screened for analgesic and antiinflammatory properties in mice and rats. The tabulated results reveal several 2-(4-substituted phenyl-1-piperazinyl)ethyl 2-(7- or 8-substituted 4-quinolinylamino)benzoates to be six to nine times more
Mostafa M Ghorab et al.
Acta pharmaceutica (Zagreb, Croatia), 64(3), 285-297 (2014-10-10)
Novel nineteen compounds based on a 4-aminoquinoline scaffold were designed and synthesized as potential antiproliferative agents. The new compounds were N-substituted at the 4-position by aryl or heteroaryl (1-9), quinolin- 3-yl (10), 2-methylquinolin-3-yl (11), thiazol-2-yl (12), and dapsone moieties (13
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service