176478
3-(4-Fluorobenzoyl)propionic acid
97%
Synonym(s):
Haloperidol metabolite III, R 11302
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
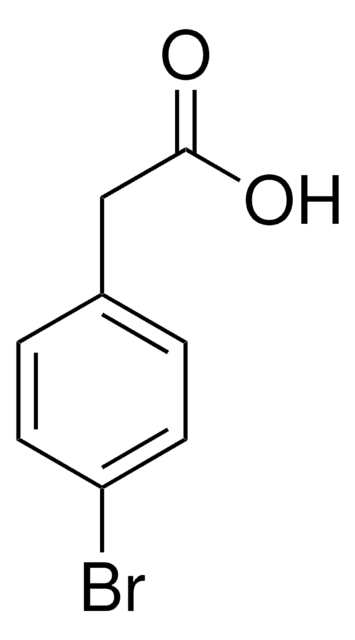
FC6H4COCH2CH2CO2H
CAS Number:
Molecular Weight:
196.18
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39040324
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
100-102 °C (lit.)
SMILES string
OC(=O)CCC(=O)c1ccc(F)cc1
InChI
1S/C10H9FO3/c11-8-3-1-7(2-4-8)9(12)5-6-10(13)14/h1-4H,5-6H2,(H,13,14)
InChI key
WUYWHIAAQYQKPP-UHFFFAOYSA-N
General description
3-(4-Fluorobenzoyl)propionic acid is a metabolite of haloperidol, a dopamine D2 receptor blocker.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sarita Forsback et al.
Synapse (New York, N.Y.), 51(2), 119-127 (2003-11-18)
In order to characterize the sensitivity of an analog of levodopa and a dopamine transporter ligand to detect defects in nigrostriatal function, the uptake of [(18)F]FDOPA and [(18)F]CFT was studied ex vivo in a rat model of Parkinson's disease. The
M Watanabe et al.
Xenobiotica; the fate of foreign compounds in biological systems, 29(8), 839-846 (1999-12-20)
1. The aim was to identify whether CYP3A metabolizes bromperidol (BP), an antipsychotic drug, to form 4-fluorobenzoyl-propionic acid (FBPA) in hepatic microsomes from 8-week-old male Sprague-Dawley rats and to investigate whether an inhibitor or an inducer of CYP3A affects BP
G A Digenis et al.
Journal of pharmaceutical sciences, 70(9), 985-989 (1981-09-01)
Tissue distribution studies of [18F]haloperidol and [82Br]bromperidol, two potent neuroleptic drugs, were performed in rats by serial sacrifice. The usefulness of external scintigraphy in obtaining tissue distribution data in large animals is demonstrated by the tissue distribution of [18F]haloperidol in
T Tateishi et al.
Life sciences, 67(24), 2913-2920 (2001-01-02)
We studied the biotransformation of haloperidol, bromperidol and their reduced forms by human liver microsomes. Nifedipine oxidation (CYP3A) activity correlated significantly with N-dealkylation rates of haloperidol and bromperidol and oxidation rates of their reduced forms, while neither ethoxyresorufin O-deethylation (CYP1A2)
M Watanabe et al.
Fundamental & clinical pharmacology, 13(3), 337-342 (1999-07-07)
Haloperidol (HP), an antipsychotic drug, is N-dealkylated by cytochrome P450 (CYP) to 4-fluorobenzoylpropionic acid (FBPA). The purpose of this study was to identify whether CYP3A metabolizes HP to FBPA in hepatic microsomes of rats and to investigate whether an inhibitor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service