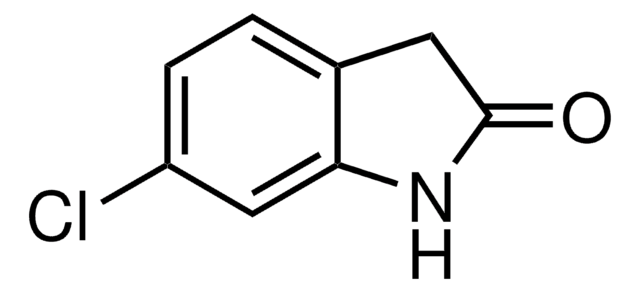
O9808
2-Oxindole
97%
Synonym(s):
2-Indolinone, Oxindole
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H7NO
CAS Number:
Molecular Weight:
133.15
Beilstein:
114692
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
crystals
bp
227 °C/73 mmHg (lit.)
mp
123-128 °C (lit.)
SMILES string
O=C1Cc2ccccc2N1
InChI
1S/C8H7NO/c10-8-5-6-3-1-2-4-7(6)9-8/h1-4H,5H2,(H,9,10)
InChI key
JYGFTBXVXVMTGB-UHFFFAOYSA-N
Gene Information
human ... PGR(5241)
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ke Shen et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(12), 3736-3742 (2010-02-23)
A direct catalytic asymmetric aldol-type reaction of 3-substituted-2-oxindoles with glyoxal derivatives and ethyl trifluoropyruvate, catalyzed by a chiral N,N'-dioxide-Sc(OTf)(3) (Tf = trifluoromethanesulfonyl) complex, has been developed that tolerates a wide range of substrates. The reaction proceeds in good yields and
G Mannaioni et al.
British journal of pharmacology, 125(8), 1751-1760 (1999-01-14)
1. The aim of the present work was to investigate the electrophysiological effects of oxindole, a tryptophan metabolite present in rat blood and brain, and recently proposed as a contributing factor in the pathogenesis of hepatic encephalopathy. 2. Using rat
Zhigang Yang et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(22), 6632-6637 (2010-04-22)
A highly enantioselective alpha-amination of 3-substituted oxindoles with azodicarboxylates catalyzed by a chiral Sc(OTf)(3)/N,N'-dioxide complex (Tf: triflate) has been developed and affords the corresponding 3-amino-2-oxindole derivatives in high yields (up to 98%) with excellent enantioselectivities (up to 99% ee). The
Junwei Wang et al.
Organic letters, 14(9), 2210-2213 (2012-04-14)
The reaction of phenyliodine bis(trifluoroacetate) (PIFA) with a series of anilides 1 (E = CO(2)Et) in CF(3)CH(2)OH was found to give 3-hydroxy-2-oxindole derivatives 2, while that with various anilides 1' (E = CON(R(4))Ar) afforded the C(2)-symmetric or unsymmetric spirooxindoles 3.
Ryo Shintani et al.
Chemical communications (Cambridge, England), 46(36), 6822-6824 (2010-08-21)
A copper-catalyzed addition of arylboronates to isatins has been developed to give 3-aryl-3-hydroxy-2-oxindoles under mild conditions. The catalytic cycle of this process has been examined through a series of stoichiometric reactions and an effective asymmetric variant has also been described
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service