902136
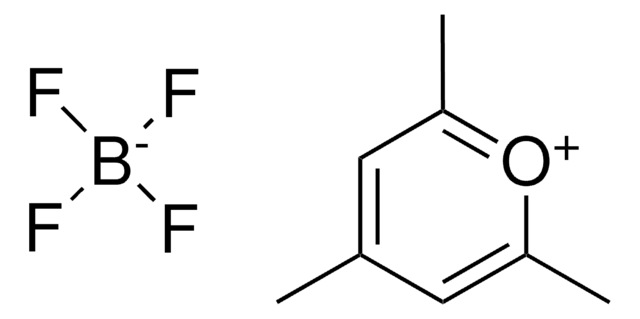
2,4,6-Tri-(4-fluorophenyl)pyrylium tetrafluoroborate
≥95%
Synonym(s):
Triphenylpyrylium photosensitizer, [T(p-F)PPT
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C23H14BF7O
CAS Number:
Molecular Weight:
450.16
UNSPSC Code:
12352101
NACRES:
NA.22
Recommended Products
Assay
≥95%
form
powder
reaction suitability
reagent type: catalyst
reaction type: Photocatalysis
mp
242-245 °C
photocatalyst activation
465 nm
Application
Triarylpyrylium salt used as a photosensitizer in photocatalysis and material science.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Antonio Franconetti et al.
Physical chemistry chemical physics : PCCP, 16(34), 18442-18453 (2014-07-30)
Noncovalent interactions of anions with electron-deficient aromatic rings that have been studied so far involve non-heteroaromatic or nitrogen-based heteroaromatic systems. Here we report the first case of an organic oxygenated aromatic system, in particular the tri-aryl-pyrylium tetrafluoroborate system, for which
Photocatalytic Cross-Dehydrogenative Amination Reactions between Phenols and Diarylamines.
Zhao Y, et al.
ACS Catalysis, 7 (4), 2446-2451 (2017)
Kuai Wang et al.
Organic letters, 19(8), 1958-1961 (2017-04-04)
A highly regioselective [2 + 2 + 2] cyclization of aromatic alkynes with nitriles is developed for the preparation of 2,3,6-trisubstituted pyridines under visible-light irradiation using a pyrylium salt as the photoredox catalyst. This cycloaddition is achieved through a photooxidative
Ji Young Cho et al.
The Journal of organic chemistry, 83(2), 805-811 (2017-12-14)
Dibenzofurans are naturally occurring molecules that have received considerable attention for a variety of practical applications, such as in pharmaceuticals and electronic materials. Herein, an efficient and eco-friendly method for the synthesis of dibenzofuran derivatives via intramolecular C-O bond formation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![Methyl 2-(9-bromo-2,3-dioxo-2,3,6,7-tetrahydro-1H,5H-pyrido[1,2,3-de]quinoxalin-5-yl)acetate ≥90%](/deepweb/assets/sigmaaldrich/product/structures/955/217/e6cb560d-1177-4dc4-b686-7c790e13f1f4/640/e6cb560d-1177-4dc4-b686-7c790e13f1f4.png)






