62262
Ethyl linoleate
technical, ≥65% (GC)
Synonym(s):
Linoleic acid ethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
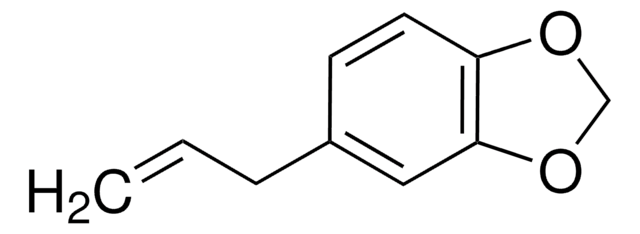
CH3(CH2)3(CH2CH=CH)2(CH2)7COOC2H5
CAS Number:
Molecular Weight:
308.50
Beilstein:
1727827
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical
Quality Level
concentration
≥65% (GC)
refractive index
n20/D 1.455 (lit.)
n20/D 1.455
bp
224 °C/17 mmHg (lit.)
density
0.876 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
CCCCC\C=C/C\C=C/CCCCCCCC(=O)OCC
InChI
1S/C20H36O2/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22-4-2/h8-9,11-12H,3-7,10,13-19H2,1-2H3/b9-8-,12-11-
InChI key
FMMOOAYVCKXGMF-MURFETPASA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ethyl linoleate can undergo auto-oxidation in the presence of the catalyst manganese(II)acetylacetonate.
Application
Ethyl linoleate can be used as a drying agent for alkyd paints. It can also form N2,3-ethenoguanine via reaction with deoxyguanosine.
Biochem/physiol Actions
Ethyl linoleate solated from Oxalis triangularis inhibits forskolin-induced melanogenesis and tyrosinase activity in mouse B16 melanoma cells . This anti-melanogenic effect is mediated by inhibiting cAMP production.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
"Fast autoxidation of ethyl linoleate catalyzed by [Mn (acac) 3] and bipyridine: A possible drying catalyst for alkyd paints"
Gorkum VR, et al.
Inorganic Chemistry, 43(08), 2456-2458 (2004)
"4-Hydroxy-2-nonenal and ethyl linoleate form N 2, 3-ethenoguanine under peroxidizing conditions"
Ham.L J-A, et al.
Chemical Research in Toxicology, 1243-1250 (2000)
A J Ham et al.
Chemical research in toxicology, 13(12), 1243-1250 (2000-12-22)
In these studies, we demonstrate that N(2),3-ethenoguanine (N(2), 3-epsilonGua) is formed from lipid peroxidation as well as other oxidative reactions. Ethyl linoleate (EtLA) or 4-hydroxy-2-nonenal (HNE) was reacted with dGuo in the presence of tert-butyl hydroperoxide (t-BuOOH) for 72 h
C F Bearer et al.
Alcoholism, clinical and experimental research, 23(3), 487-493 (1999-04-09)
Fetal alcohol syndrome, fetal alcohol effects, alcohol-related neurodevelopmental disorder, and alcohol-related birth defects, all terms referring to the spectrum of consequences of in utero exposure to ethanol, are a major public health burden. There is currently no laboratory test to
B L Hungund et al.
Alcoholism, clinical and experimental research, 19(2), 374-377 (1995-04-01)
The fate of [14C]ethyl-linoleate (EthLin) after its intravenous administration was investigated in pentobarbital-anesthetized rats. The disappearance of [14C]EthLin from the plasma was very rapid and followed quite closely a biexponential function of time. Fitting of the experimental data to a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service