54345
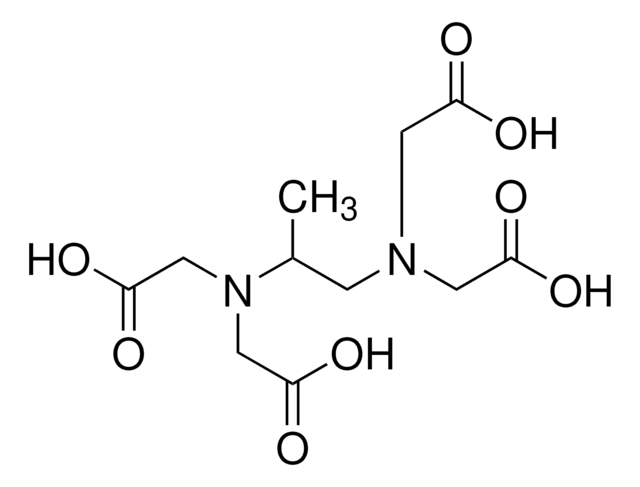
N-(2-Hydroxyethyl)iminodiacetic acid
≥98.0% (T)
Synonym(s):
Ethanol diglycine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOCH2CH2N(CH2COOH)2
CAS Number:
Molecular Weight:
177.16
Beilstein:
1780628
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (T)
form
solid
mp
178 °C (lit.)
application(s)
peptide synthesis
SMILES string
OCCN(CC(O)=O)CC(O)=O
InChI
1S/C6H11NO5/c8-2-1-7(3-5(9)10)4-6(11)12/h8H,1-4H2,(H,9,10)(H,11,12)
InChI key
JYXGIOKAKDAARW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-(2-Hydroxyethyl)iminodiacetic acid (HEIDA) is a biodegradable and strong metal chelating agent, similar to nitrilotriacetic acid (NTA).
Application
N-(2-Hydroxyethyl)iminodiacetic acid (HEIDA) can be used in the following processes:
- HEIDA can be used as a metal chelating agent for Fe(III) ion. The presence of HEIDA improves the Fenton′s destruction performance of PCE (perchloroethylene) existing as dense non-aqueous phase liquid (DNAPL) in soil slurry systems.
- Oxorhenium(V) complexes with HEIDA are used for the carboxylation of ethane by CO, with potassium peroxodisulfate (K2S2O8)/trifluoroacetic acid (TFA), to afford propionic and acetic acid in good yield.
- Vanadium complexes with HEIDA are used for the peroxidative hydroxylation of benzene and oxidation of mesitylene.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Single-Pot Ethane Carboxylation Catalyzed by New Oxorhenium (V) Complexes with N, O Ligands.
Kirillov AM, et al.
advanced synthesis and catalysis, 347(10), 1435-1446 (2005)
Single-Pot Ethane Carboxylation Catalyzed by New Oxorhenium (V) Complexes with N, O Ligands.
Kirillov AM, et al.
Advanced Synthesis & Catalysis, 347(10), 1435-1446 (2005)
Peroxidative oxidation of benzene and mesitylene by vanadium catalysts.
Reis PM, et al.
J. Mol. Catal. A: Chem., 224(1-2), 189-195 (2004)
Namgoo Kang et al.
Chemosphere, 63(10), 1685-1698 (2005-12-06)
The Fenton's system is applied to the destruction of perchloroethylene (PCE) present as a dense non-aqueous phase liquid (DNAPL) in soil slurry systems; the initial concentration of PCE was 45 times higher than its aqueous solubility. Studies were conducted in
Namgoo Kang et al.
Chemosphere, 61(7), 909-922 (2005-11-01)
Fenton's destruction of benzene, toluene, ethylbenzene, and xylene (BTEX) was investigated in soil slurry batch reactors. The purpose of the investigation was to quantify the enhancement of oxidation rates and efficiency by varying process conditions such as iron catalyst (Fe(II)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service