473790
trans-2-Phenylvinylboronic acid
97%
Synonym(s):
(E)-2-phenyl-Etheneboronic acid, (E)-Phenylethenylboronic acid, (E)-Styreneboronic acid, (E)-Styrylboronic acid, trans-(2-Phenylethenyl)boronic acid, trans-Phenylvinyl boronic acid
About This Item
Recommended Products
Quality Level
Assay
97%
mp
146-156 °C (lit.)
SMILES string
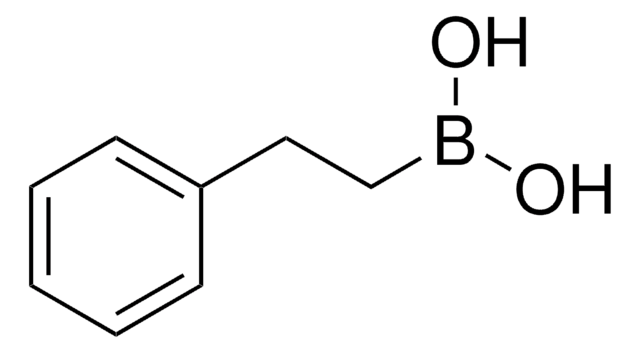
OB(O)\C=C\c1ccccc1
InChI
1S/C8H9BO2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7,10-11H/b7-6+
InChI key
VKIJXFIYBAYHOE-VOTSOKGWSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Palladium (Pd)-catalyzed Suzuki-Miyaura coupling reactions
- Rhodium (Rh)-catalyzed intramolecular amination of aryl azides
- Diastereoselective synthesis via Pd-catalyzed Heck-Suzuki cascade reaction
- Copper (Cu)-mediated cyanation
- Rhodium (Rh)-catalyzed asymmetric addition
- Diastereoselective synthesis via iridium (Ir)-catalyzed addition
- Palladium (Pd)-catalyzed cascade cyclization
Reagent used in Preparation of
- Optically active unsaturated amino acids by diastereoselective Petasis borono-Mannich reaction
- Amino alcohol dienes via Petasis 3-component reaction using Ru-catalyzed ring-closing metathesis and isomerization
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)