All Photos(1)
About This Item
Linear Formula:
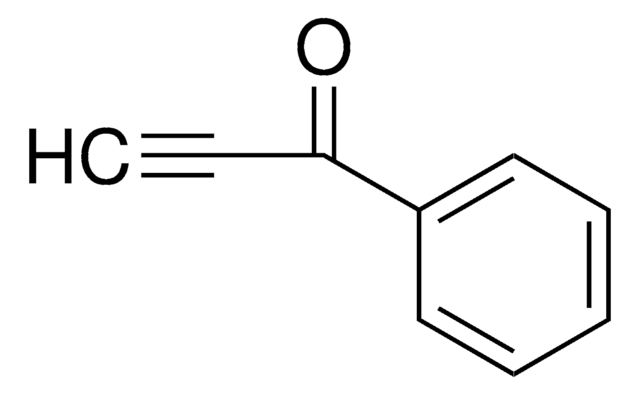
HC≡CCOCH3
CAS Number:
Molecular Weight:
68.07
Beilstein:
605353
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.406 (lit.)
bp
85 °C (lit.)
density
0.87 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
CC(=O)C#C
InChI
1S/C4H4O/c1-3-4(2)5/h1H,2H3
InChI key
XRGPFNGLRSIPSA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Butyn-2-one undergoes asymmetric double-Michael reaction with ortho-tosylamidophenyl malonate catalyzed by chiral aminophosphines to yield indolines. It undergoes double Michael reaction with nitrogen-containing tethered diacid to give pipecolic acid derivatives.
Application
3-Butyn-2-one was used in the synthesis of clerodane diterpenoid (+/-)-sacacarin. It was used as substrate in stereoselective, conjugate arylation mediated by gallium(III) chloride leading to (E)-α,β-unsaturated ketones.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
30.2 °F - closed cup
Flash Point(C)
-1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R B Grossman et al.
Organic letters, 3(25), 4027-4030 (2001-12-12)
[reaction: see text] The putative structure of the naturally occurring clerodane diterpenoid (+/-)-sacacarin has been prepared in only 10 steps, six of which are C-C bond-forming steps, in a chemo-, regio-, and diastereoselective manner. The key part of the synthesis
Synlett, 809-809 (2007)
F Hughes et al.
Organic letters, 3(18), 2911-2914 (2001-09-01)
[reaction: see text]. Nitrogen-containing tethered diacids, easily prepared by reductive alkylation of diethyl aminomalonate or ethyl cyanoglycinate, undergo double Michael reactions with 3-butyn-2-one to give highly functionalized and substituted piperidines (pipecolic acid derivatives) with surprisingly high stereoselectivity. The heterocyclic double
San N Khong et al.
Molecules (Basel, Switzerland), 17(5), 5626-5650 (2012-05-15)
The bisphosphine-catalyzed double-Michael addition of dinucleophiles to electron-deficient acetylenes is an efficient process for the synthesis of many nitrogen-containing heterocycles. Because the resulting heterocycles contain at least one stereogenic center, this double-Michael reaction would be even more useful if an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service