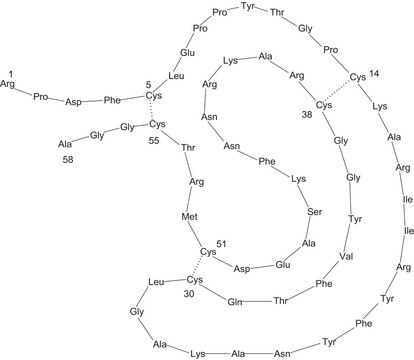
SRP6313
Alpha 2 Antiplasmin from human plasma
≥95% (SDS-PAGE), lyophilized
Synonym(s):
AAP, Alpha-2-plasmin inhibitor, PLI, SERPINF2
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352202
NACRES:
NA.32
Recommended Products
product name
Alpha 2 Antiplasmin from human plasma, ≥95% (SDS-PAGE)
biological source
human
Assay
≥95% (SDS-PAGE)
form
lyophilized
potency
≥5.0 I.U. per mg
mol wt
70 kDa
packaging
pkg of 100 μg
UniProt accession no.
shipped in
wet ice
storage temp.
−20°C
Gene Information
human ... SERPINF2(5345)
General description
α-2 antiplasmin (AAP) is a member of the Serpin superfamily. Liver and kidney are major sites of its production and other tissues such as muscle, intestine, central nervous system, and placenta also express its mRNA at a moderate level. The tissue expression pattern indicates that it is a key regulator of plasmin mediated proteolysis in these tissues. The gene encoding this protein is localized on human chromosome 17.
α-2 antiplasmin (AAP) is a member of the Serpin superfamily. Liver and kidney are major sites of its production and other tissues such as muscle, intestine, central nervous system, and placenta also express its mRNA at a moderate level. The tissue expression pattern indicates that it is a key regulator of plasmin mediated proteolysis in these tissues. The AAP gene is mapped to human chromosome 17p13 and codes for a glycoprotein of single chain containing 464 amino acid residues.
Biochem/physiol Actions
α-2 antiplasmin (AAP) is the primary physiological inhibitor of the serine protease plasmin, which is responsible for the dissolution of fibrin clots. In addition to plasmin, it is also an efficient inhibitor of trypsin and chymotrypsin. α-antiplasmin-deficiency is a rare coagulation disorder which allows unrestrained fibrinolytic activity. Individuals with this condition may receive therapeutic A2AP prior to surgery to prevent postoperative hemorrhaging.
α-2 antiplasmin (AAP) is the primary physiological inhibitor of the serine protease plasmin, which is responsible for the dissolution of fibrin clots. It inhibits the action of protease by the formation In addition to plasmin, it is also an efficient inhibitor of trypsin and chymotrypsin. α-antiplasmin-deficiency is a rare coagulation disorder which allows unrestrained fibrinolytic activity. AAP is known to inhibit fibrinogenolysis by preventing free plasmin circulation.
Physical form
Lyophilized from 20 mM Bis-Tris, pH 6.4, with 200 mM NaCl.
Reconstitution
In water or aqueous buffer
Disclaimer
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Noncovalent interaction of ?2-antiplasmin with fibrin (ogen): localization of ?2-antiplasmin-binding sites.
Tsurupa G, et al.
Biochemistry, 49(35), 7643-7651 (2010)
Genome-wide loss of heterozygosity and copy number alteration in esophageal squamous cell carcinoma using the Affymetrix GeneChip Mapping 10 K array.
Hu N
BMC Genomics, 7, 299-299 (2006)
Open-heart surgery in a patient with heterozygous alpha 2-antiplasmin deficiency. Perioperative strategies in the first reported case.
Shahian DM and Levine JD
Chest, 97(6), 1488-1490 (1990)
Ekaterina Mindel et al.
Journal of neuroscience research, 99(3), 966-976 (2020-12-10)
Many coagulation factor proteases are increased in the brain during ischemic stroke. One of these proteases is plasmin. In this study we established a novel method for direct quantitative measurement of plasmin activity in male mouse brain slices using a
The kidney is a major site of alpha(2)-antiplasmin production.
Menoud PA
The Journal of Clinical Investigation, 97(11), 2478-2484 (1996)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service