About This Item
Recommended Products
Quality Level
Assay
≥97% (HPLC)
form
powder
UniProt accession no.
storage temp.
−20°C
SMILES string
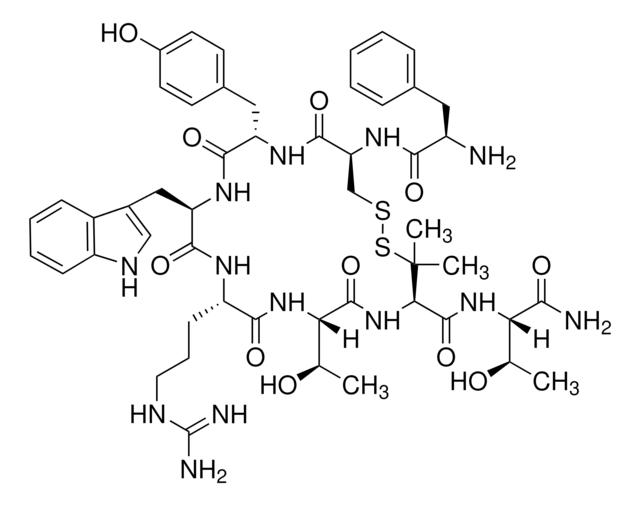
C[C@@H](O)[C@H](NC(=O)[C@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc4ccc(O)cc4)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@H](N)Cc5ccccc5)[C@@H](C)O)C(N)=O
InChI
1S/C50H67N11O11S2/c1-26(62)39(42(53)65)59-49(72)41-50(3,4)74-73-25-38(58-43(66)33(52)21-28-11-6-5-7-12-28)47(70)56-36(22-29-16-18-31(64)19-17-29)45(68)57-37(23-30-24-54-34-14-9-8-13-32(30)34)46(69)55-35(15-10-20-51)44(67)60-40(27(2)63)48(71)61-41/h5-9,11-14,16-19,24,26-27,33,35-41,54,62-64H,10,15,20-23,25,51-52H2,1-4H3,(H2,53,65)(H,55,69)(H,56,70)(H,57,68)(H,58,66)(H,59,72)(H,60,67)(H,61,71)/t26-,27-,33-,35+,36+,37-,38+,39+,40+,41-/m1/s1
InChI key
PZWWYAHWHHNCHO-FGHAYEPSSA-N
Gene Information
mouse ... Pnoc(18155)
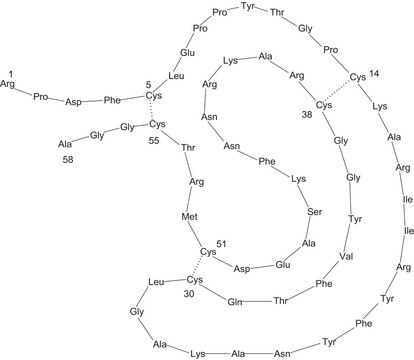
Amino Acid Sequence
General description
Application
- to study the anxiogenic effects induced by CTOP in mice and rat.
- to study the effect of μ-opioid antagonist, CTOP on the bovine milk-derived LF (BLF)-induced analgesia.
- to determine whether μ-opioid receptors act cooperatively with 5-hydroxytryptamine (5-HT1A) receptors to regulate the behaviors generated in the elevated T-maze (ETM).
Biochem/physiol Actions
Linkage
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service