All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H15N
CAS Number:
Molecular Weight:
161.24
Beilstein:
124508
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
61-65 °C (lit.)
SMILES string
C1CC(CCN1)c2ccccc2
InChI
1S/C11H15N/c1-2-4-10(5-3-1)11-6-8-12-9-7-11/h1-5,11-12H,6-9H2
InChI key
UTBULQCHEUWJNV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P Singh et al.
Journal of enzyme inhibition, 16(4), 331-338 (2002-03-28)
Two series of compounds were recently reported as novel alpha1a-selective adrenoceptor antagonists. In the first series, a dihydropyrimidone moiety is attached to a 4-phenyl piperidine containing side chain, while in the second, it is linked to a 4-substituted phenyl piperazine
Xing-hai Wang et al.
European journal of medicinal chemistry, 41(2), 226-232 (2006-01-13)
A nonlinear QSAR study was conducted on a series of 4-phenylpiperidine derivatives (4PPs) acting as mu opioid agonists by three-layer back-propagation neural network (NN) method. At first a variety of molecular descriptors were calculated and then selected with two-stage least
Clandestine drug synthesis.
W H Soine
Medicinal research reviews, 6(1), 41-74 (1986-01-01)
Diane K Luci et al.
Bioorganic & medicinal chemistry letters, 17(23), 6489-6492 (2007-10-16)
Various 4-phenylpiperidine-benzoxazin-3-ones were synthesized and biologically evaluated as urotensin-II (U-II) receptor antagonists. Compound 12i was identified from in vitro evaluation as a low nanomolar antagonist against both rat and human U-II receptors. This compound showed in vivo efficacy in reversing
A G Ishkov et al.
Voprosy meditsinskoi khimii, 38(2), 25-28 (1992-03-01)
A rate of utilization of 4-phenyl piperidine and its 12 derivatives by brain monoamine oxidase (MAO) was studied as compared with typical neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). The enzyme was isolated from P2 synaptosomal fraction of brain corpus striatum of Sprague-Dawley rats.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service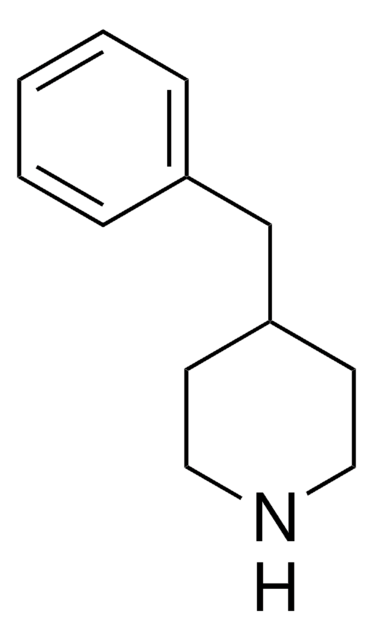





![Benzo[b]thien-2-ylboronic acid ≥95%](/deepweb/assets/sigmaaldrich/product/structures/251/077/d0ead874-b533-4dcb-890d-8816a0018ccd/640/d0ead874-b533-4dcb-890d-8816a0018ccd.png)


![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)

