597988
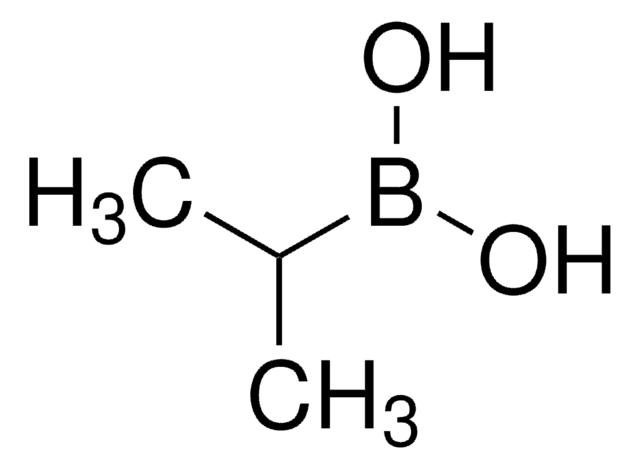
Cyclopropylboronic acid
Synonym(s):
Cyclopropaneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C3H7BO2
CAS Number:
Molecular Weight:
85.90
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
Quality Level
mp
90-95 °C (lit.)
storage temp.
−20°C
SMILES string
OB(O)C1CC1
InChI
1S/C3H7BO2/c5-4(6)3-1-2-3/h3,5-6H,1-2H2
InChI key
WLVKDFJTYKELLQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Boronic acid component in a study of carbon-hydrogen bond alkylation in the presence of Pd(II), Ag(I) and benzoquinone.
Cu-mediated N-cyclopropanation
Reagent used for
Reagent used in Preparation of
- Microwave-assisted copper(II)-catalyzed N-cyclopropylation
- Nickel- and copper-catalyzed Suzuki-Miyaura coupling reaction of arenes
- Palladacycle-catalyzed Suzuki-cross coupling of aryl halides with cyclopropylboronic acid
- Palladium(0)-catalyzed cyclopropane C-H bond functionalization
- Palladium-catalyzed decarboxylative coupling
- Palladium-catalyzed ligand-directed oxidative functionalization of cyclopropanes
- Palladium-catalyzed Suzuki coupling reaction
Reagent used in Preparation of
- Diaryl ketones by arylation of arylboronic acids with aromatic aldehydes catalyzed by Cu(OTf)2 and Xantphos
- Aminothiazolylpyrrolidine-based tartrate diamides as TACE inhibitors to treat inflammatory disorders and cancer
Other Notes
Contains varying amounts of anhydride
May contain 3-5% cyclopropanol
May contain 5-10% boric acid
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chaoyang Dai et al.
Bioorganic & medicinal chemistry letters, 21(10), 3172-3176 (2011-04-05)
TNF-α converting enzyme (TACE) inhibitors are promising agents to treat inflammatory disorders and cancer. We have investigated novel tartrate diamide TACE inhibitors where the tartrate core binds to zinc in a unique tridentate fashion. Incorporating (R)-2-(2-N-alkylaminothiazol-4-yl)pyrrolidines into the left hand
Microwave-assisted N-cyclopropylation of pyridinols with cyclopropyl boronic acid
Tambe, Y. B.; et al.
Synthetic Communications, 42, 1341-1348 (2012)
Facile synthesis of aryl(het)cyclopropane catalyzed by palladacycle
Zhang, M.; et al.
Tetrahedron, 68, 900-905 (2012)
A Mild Palladium-Catalyzed Suzuki Coupling Reaction of Quinoline Carboxylates with Boronic Acids
W. Li, et al.,
Advanced Synthesis & Catalysis, 353, 1671-1675 (2011)
Xiao Chen et al.
Journal of the American Chemical Society, 128(39), 12634-12635 (2006-09-28)
Palladium-catalyzed alkylations of sp2 and sp3 C-H bonds with either methylboroxine or alkylboronic acids were developed. Ag2O or AgCO3 is used as a crucial oxidant and promoter for the transmetalation step. Ether, ester, alcohol, and alkene functional groups are tolerated.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)