All Photos(1)
About This Item
Empirical Formula (Hill Notation):
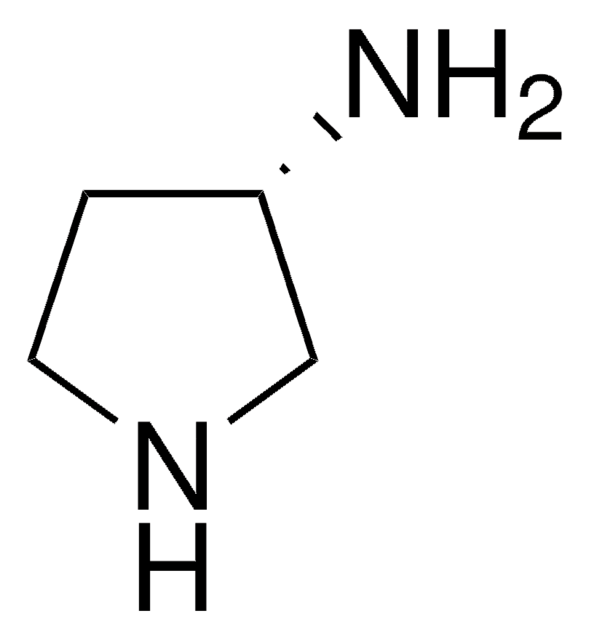
C4H10N2 · 2HCl
CAS Number:
Molecular Weight:
159.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
>300 °C (lit.)
SMILES string
Cl[H].Cl[H].NC1CCNC1
InChI
1S/C4H10N2.2ClH/c5-4-1-2-6-3-4;;/h4,6H,1-3,5H2;2*1H
InChI key
NJPNCMOUEXEGBL-UHFFFAOYSA-N
General description
3-Aminopyrrolidine dihydrochloride (3-pyrrolidinamine dihydrochloride) is one of the key intermediate of tosufloxacin and other quinolone antibiotics.
Application
3-Aminopyrrolidine dihydrochloride is the suitable reagent used as an internal standard for the quantitative analysis of amino acids in bio-fluids. (R,S) 3-Aminopyrrolidine dihydrochloride may be used in the preparation of cis-[PdCl2(pyrr)] and cis-[PtCl2(pyrr)] (pyrr= (R,S)-3-aminopyrrolidine) complexes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Pd (II) and Pt (II)(R, S)-3-aminopyrrolidine complexes. Reactions with 9-ethylguanine and study of their antiproliferative activity.
Riera X, et al.
Inorgorganica Chimica Acta, 339, 253-264 (2002)
Synthesis of 3-Aminopyrrolidine and its N-alkylating Derivatives [J].
Run-pu SHEN, et al.
Journal of Chemical Engineering of Chinese Universities / Gao Xiao Hua Xue Gong Cheng Xue Bao, 4, 014-014 (2003)
Jieyu Zhao et al.
Journal of proteome research, 15(2), 468-476 (2016-01-20)
The interaction between carbon (C) and nitrogen (N) metabolism can reflect plant growth status and environmental factors. Little is known regarding the connections between C-N metabolism and growing regions under field conditions. To comprehensively investigate the relationship in mature tobacco
Yukino Ogawa et al.
Scientific reports, 10(1), 19554-19554 (2020-11-13)
Dysbiosis of the gut microbiota affects physiological processes, including brain functions, by altering the intestinal metabolism. Here we examined the effects of the gut microbiota on sleep/wake regulation. C57BL/6 male mice were treated with broad-spectrum antibiotics for 4 weeks to deplete
Tomoyo Furukawa et al.
The International journal of developmental biology, 63(1-2), 37-43 (2019-03-29)
The receptors of gamma-aminobutyric acid (GABA), which is a well-known neurotransmitter, are expressed in the anterior-to-mid neural tube at an early stage of Xenopus development, but there has been no report on the role of GABA in the presumptive central
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service