268674
2-Aminoethylphosphonic acid
99%
Synonym(s):
2-AEP
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
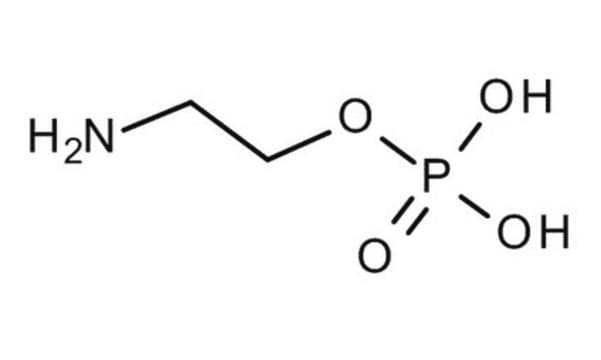
H2NCH2CH2P(O)(OH)2
CAS Number:
Molecular Weight:
125.06
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
296 °C (dec.) (lit.)
solubility
water: soluble 50 mg/mL, clear, colorless
functional group
amine
SMILES string
NCCP(O)(O)=O
InChI
1S/C2H8NO3P/c3-1-2-7(4,5)6/h1-3H2,(H2,4,5,6)
InChI key
QQVDJLLNRSOCEL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The surface of gold nanoparticles (Au NPs) were functionalized with 2-aminoethylphosphonic acid that exhibited calcium affinity which enabled targeted delivery of Au NPs to calcified tissue.
Application
2-Aminoethylphosphonic acid was used as a growth medium for the marine bacterium Roseovarius nubinhibens ISM.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Magdalena Klimek-Ochab et al.
Biodegradation, 18(2), 223-231 (2006-06-08)
Air-born mixed fungal and bacterial culture capable of complete degradation of ciliatine was isolated. The utilization of the natural organophosphonate proceeded in the phosphate independent manner. Enzymatic activity involved in ciliatine degradation studied in the fungal cell-free extract proved to
Synthesis of monensin derivatives and their effect on the activity of ricin A-chain immunotoxins.
M Colombatti et al.
Methods in molecular biology (Clifton, N.J.), 166, 55-70 (2001-02-24)
Celia C H Chen et al.
Biochemistry, 41(44), 13162-13169 (2002-10-31)
Phosphonates allow certain organisms to thrive in otherwise hostile environments, and 2-aminoethylphosphonate (AEP) is a precursor of many cellular phosphonates. AEP transaminase (AEPT) is an enzyme essential to phosphonate synthesis and degradation pathways. The crystal structure of AEP transaminase was
Zhiqiang Xu et al.
Chemical communications (Cambridge, England), (7)(7), 878-879 (2003-05-13)
Carbon-supported catalysts were phosphonated using 2-aminoethylphosphonic acid, and the resulting catalysts with largely enhanced proton conductivity performed substantially better than the untreated counterparts in proton-exchange membrane fuel cells.
Thomas Danhorn et al.
Journal of bacteriology, 186(14), 4492-4501 (2004-07-03)
The plant pathogen Agrobacterium tumefaciens forms architecturally complex biofilms on inert surfaces. Adherence of A. tumefaciens C58 was significantly enhanced under phosphate limitation compared to phosphate-replete conditions, despite slower overall growth under low-phosphate conditions. Replacement of Pi with sn-glycerol-3-phosphate and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service