186171
n-Butyllithium solution
1.6 M in hexanes
Synonym(s):
n-BuLi, Butyl lithium, Butyllithium solution, Lithium-1-butanide
About This Item
Recommended Products
form
liquid
Quality Level
concentration
1.6 M in hexanes
density
0.68 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
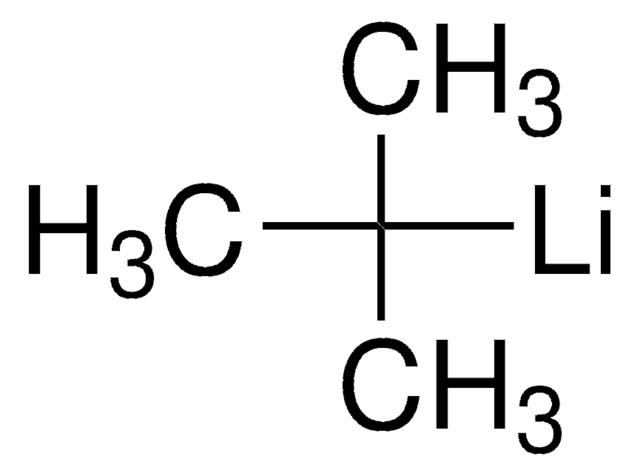
[Li]CCCC
InChI
1S/C4H9.Li/c1-3-4-2;/h1,3-4H2,2H3;
InChI key
MZRVEZGGRBJDDB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Copolymerization of butadiene and styrene under the influence of n-butyllithium: This study explores the copolymerization process using n-butyllithium as a catalyst, which is significant for the development of new synthetic rubber materials (V Bronskaya et al., 2023).
- Modifiers of n-Butyllithium in the Synthesis of Polybutadiene and Styrene-Butadiene Rubbers: Explores the use of n-butyllithium with different modifiers in the synthesis of industrial rubbers, important for understanding the control of polymer properties (VS Glukhovskoi et al., 2014).
- Aggregation and Solvation of n-Butyllithium: Examines the aggregation behavior and solvation properties of n-butyllithium in various solvents, providing insights into its chemical interactions essential for organic synthesis (O Tai et al., 2017).
Packaging
The 25 mL Sure/Seal™ bottle is recommended as a single-use bottle. Repeated punctures will likely result in decreased performance of product.
Other Notes
Legal Information
related product
Signal Word
Danger
Hazard Statements
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Repr. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
Target Organs
Central nervous system
Supplementary Hazards
Storage Class Code
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 3
Flash Point(F)
-14.8 °F - closed cup
Flash Point(C)
-26 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service