76547
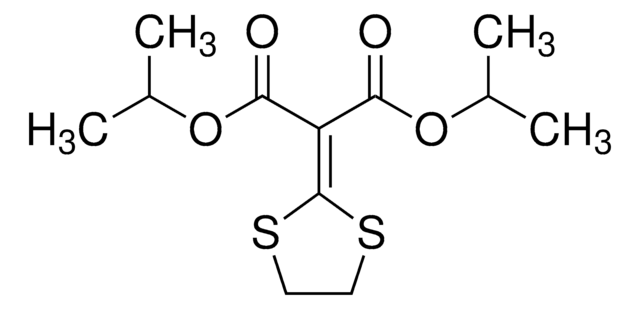
Isoprothiolane
PESTANAL®, analytical standard
Synonym(s):
Diisopropyl 2-(1,3-dithiolan-2-ylidene)malonate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H18O4S2
CAS Number:
Molecular Weight:
290.40
Beilstein:
2128528
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
product line
PESTANAL®
Assay
≥98.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
agriculture
environmental
format
neat
storage temp.
2-8°C
SMILES string
CC(C)OC(=O)\C(C(=O)OC(C)C)=C1/SCCS1
InChI
1S/C12H18O4S2/c1-7(2)15-10(13)9(11(14)16-8(3)4)12-17-5-6-18-12/h7-8H,5-6H2,1-4H3
InChI key
UFHLMYOGRXOCSL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Isoprothiolane is a systemic fungicide, which is used in controlling diseases rice stem rot, rice blast and Fusarium leaf spot on rice. Its mode of action basically involves the inhibition of penetration and elongation of infecting hyphae via inhibiting the formation of infecting peg or cellulose secretion.
Application
Isoprothiolane may be used as a reference standard in the determination of isoprothiolane in food samples using gas chromatography coupled with mass spectrometry (GC-MS).
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Legal Information
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Y Ishida et al.
Physiology & behavior, 60(2), 633-638 (1996-08-01)
Responses of three chemical senses, olfaction, taste, and pit organ sense, to three pesticides were studied electrophysiologically in carp, Cyprinus carpio. Only olfaction was responsive to the three pesticides at behavioral avoidance levels, which were determined in a previous study.
H Okada et al.
The Journal of veterinary medical science, 61(5), 553-556 (1999-06-24)
This study was performed to investigate the effects of isoprothiolane on cell growth and the production of interleukin (IL)-1 and IL-6 by bovine mammary epithelial cells in vitro. Isoprothiolane increased proliferation of mammary epithelial cells in a dose-dependent manner at
Z Z Zhang et al.
Hua xi yi ke da xue xue bao = Journal of West China University of Medical Sciences = Huaxi yike daxue xuebao, 20(1), 96-98 (1989-03-01)
In this paper, the early passage diploid Syrian hamster embryo (SHE) cells were used as the source of target. Four chemicals were appraised in SHE transformation test to determine whether they were carcinogens or not. They were (1) 2-benzoyl-hydrazono-1,3-diethiolane(BHD) (technical
A Arul Selvi et al.
Journal of immunoassay & immunochemistry, 34(2), 149-165 (2013-03-30)
A simple competitive immunoassay was developed for the measurement of isoprothiolane in rice, soil, and water samples. It employed the avian antibodies (IgY) that recognized isoprothiolane as a capture reagent and isoprothiolane-alkaline phosphatase conjugate as an enzyme label. The assay
Dongli Tong et al.
Chemosphere, 230, 84-91 (2019-05-19)
Low-cost magnesium- and/or carbon-based materials have a great potential to remove soluble contaminants from surface and ground water. This study examined mechanisms that control the removal of nitrate, phosphate and pesticides (tricyclazole, malathion and isoprothiolane) during their transport through calcined
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service