PF024
MMP-9, Active, Human, Recombinant
Synonym(s):
Gelatinase B, 83 kDa Gelatinase, Matrix Metalloproteinase 9, Matrix Metalloproteinase 9, Gelatinase B, 83 kDa Gelatinase
About This Item
Recommended Products
Assay
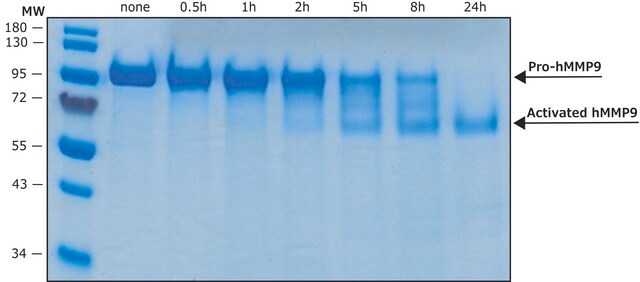
≥90% (SDS-PAGE)
Quality Level
form
liquid
specific activity
≥8.0 ΔA405/h-μg protein (thiopeptide hydrolysis assay)
does not contain
preservative
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
avoid repeated freeze/thaw cycles
shipped in
wet ice
storage temp.
−70°C
General description
Regulation of MMP activity can occur at the level of gene expression, including transcription and translation, level of activation, or at the level of inhibition by TIMPs. Thus, perturbations at any of these points can theoretically lead to alterations in ECM turnover. Expression is under tight control by pro- and anti-inflammatory cytokines and/or growth factors and, once produced the enzymes are usually secreted as inactive zymograms. Upon activation (removal of the inhibitory propeptide region of the molecules) MMPs are subject to control by locally produced TIMPs. All MMPs can be activated in vitro with organomercurial compounds (e.g. 4-aminophenylmercuric acetate), but the agents responsible for the physiological activation of all MMPs have not been clearly defined. Numerous studies indicate that members of the MMP family have the ability to activate one another. The activation of the MMPs in vivo is likely to be a critical step in terms of their biological behavior, because it is this activation that will tip the balance in favor of ECM degradation. The hallmark of diseases involving MMPs appear to be stoichiometric imbalance between active MMPs and TIMPs, leading to excessive tissue disruption and often degradation. Determination of the mechanisms that control this imbalance may open up some important therapeutic options of specific enzyme inhibitors.
Packaging
Warning
Physical form
Reconstitution
Other Notes
Backstrom, J.R., et al. 1996. J. Neuro.16, 7910.
Lim, G.P., et al. 1996. J. Neurochem.67, 251.
Xia, T., et al. 1996. Biochim. Biophys. Acta1293, 259.
Sang, Q.X., et al. 1995. Biochim. Biophys. Acta1251, 99.
Zempo, N., et al. 1994. J. Vasc. Surg.20, 217.
Birkedal-Hansen, H. 1993. J. Periodontol.64, 484.
Stetler-Stevenson, W.G., et al. 1993. FASEB J.7, 1434.
Jeffrey, J.J. 1991. Semin. Perinatol.15, 118.
Liotta, L.A., et al. 1991. Cell64, 327.
Harris, E. 1990. N. Engl. J. Med.322, 1277.
Legal Information
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service