N26806
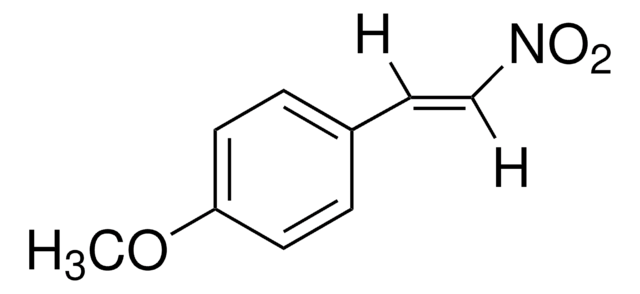
trans-β-Nitrostyrene
99%
Synonym(s):
trans-beta-Nitrostyrene, trans-1-Nitro-2-phenylethylene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH=CHNO2
CAS Number:
Molecular Weight:
149.15
Beilstein:
1210066
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
bp
250-260 °C (lit.)
mp
55-58 °C (lit.)
storage temp.
2-8°C
SMILES string
[O-][N+](=O)\C=C\c1ccccc1
InChI
1S/C8H7NO2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7H/b7-6+
InChI key
PIAOLBVUVDXHHL-VOTSOKGWSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Bridging between organocatalysis and biocatalysis: asymmetric addition of acetaldehyde to β-nitrostyrenes catalyzed by a promiscuous proline-based tautomerase.
Ellen Zandvoort et al.
Angewandte Chemie (International ed. in English), 51(5), 1240-1243 (2011-12-23)
Yufeng Miao et al.
Chembiochem : a European journal of chemical biology, 14(2), 191-194 (2013-01-11)
Exploiting catalytic promiscuity: The proline-based enzyme 4-oxalocrotonate tautomerase (4-OT) promiscuously catalyzes asymmetric Michael-type additions of linear aldehydes--ranging from acetaldehyde to octanal--to trans-β-nitrostyrene in aqueous solvent. The presence of 1.4 mol% of 4-OT effected formation of the anticipated γ-nitroaldehydes in fair
Jens Martin Werner et al.
Journal of pharmacological and toxicological methods, 57(2), 131-137 (2007-12-19)
Induction of apoptosis is perceived as the main intention of drug regimens for tumour therapy. Thus, the concentration- and time-dependence of drug-induced apoptosis should be carefully evaluated for experimental as well as for standard anti-tumour agents. A main feature of
Hui Yang et al.
Organic & biomolecular chemistry, 10(16), 3229-3235 (2012-03-10)
(S)-Proline-catalyzed nitro-Michael additions of aldehydes and ketones to β-nitrostyrene were investigated computationally (MP2/6-311+G**//M06-2X/6-31G**). Contrary to what is usually assumed in organocatalysis, the lowest-energy transition states of proline-catalyzed nitro-Michael reactions do not necessarily involve the carboxylic acid group of the proline
Junguk Park et al.
Biochemistry, 43(47), 15014-15021 (2004-11-24)
Protein tyrosine phosphatases (PTPs) catalyze the hydrolysis of phosphotyrosyl (pY) proteins to produce tyrosyl proteins and inorganic phosphate. Specific PTPs inhibitors provide useful tools for studying PTP function in signal transduction processes and potential treatment for human diseases such as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service