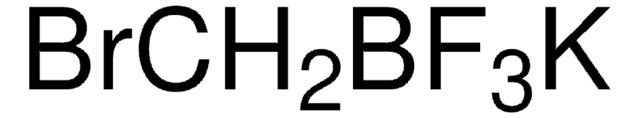About This Item
Recommended Products
form
solid
mp
245-258 °C (dec.)
functional group
iodo
SMILES string
[K+].C[B-](C)(C)CI
InChI
1S/C4H11BI.K/c1-5(2,3)4-6;/h4H2,1-3H3;/q-1;+1
InChI key
YPGDEVDDTXBNRH-UHFFFAOYSA-N
Related Categories
Application
Organotrifluoroboronates as versatile and stable boronic acid surrogates.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Bench-stable Potassium Organotrifluoroborates enable diverse C-C bond formation reactions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service