549053
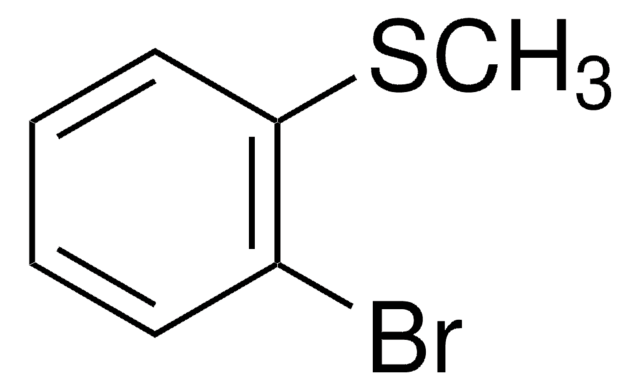
3-Bromothioanisole
97%
Synonym(s):
2-Bromophenyl methyl sulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H4SCH3
CAS Number:
Molecular Weight:
203.10
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.628 (lit.)
bp
124-125 °C/10 mmHg (lit.)
density
1.51 g/mL at 25 °C (lit.)
functional group
bromo
thioether
SMILES string
CSc1cccc(Br)c1
InChI
1S/C7H7BrS/c1-9-7-4-2-3-6(8)5-7/h2-5H,1H3
InChI key
NKYFJZAKUPSUSH-UHFFFAOYSA-N
Related Categories
General description
3-Bromothioanisole is a 3-halothioanisole derivative that is mainly used in the preparation of photoacids. It can be prepared from 3-bromobenzenethiol.
Application
3-Bromothioanisole may be used in the preparation of:
- 3-methylthiotriphenylamine
- 4-ethoxy-3′-methylthiostilbene
- 3-bromophenyl phenyl sulfide
- 9-substituted, 3,6-dithiomethylfluorenes
- 4-methoxy-3′-(methylthio)-1,1′-biphenyl
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A Preparatively Convenient Ligand-Free Catalytic PEG 2000 Suzuki- Miyaura Coupling.
Razler TM, et al.
The Journal of Organic Chemistry, 74(3), 1381-1384 (2008)
Enhancement of acid photogeneration through a para-to-meta substitution strategy in a sulfonium-based alkoxystilbene designed for two-photon polymerization.
Xia R, et al.
Chemistry of Materials, 24(2), 237-244 (2012)
Efficient photoacids based upon triarylamine dialkylsulfonium salts.
Zhou W, et al.
Journal of the American Chemical Society, 124(9), 1897-1901 (2002)
Evaluating atomic components in fluorene wires.
Klausen RS, et al.
Chemical Science, 5(4), 1561-1564 (2014)
Transition-Metal-Free Acid-Mediated Synthesis of Aryl Sulfides from Thiols and Thioethers.
Wagner AM and Sanford MS.
The Journal of Organic Chemistry, 79(5), 2263-2267 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service