494992
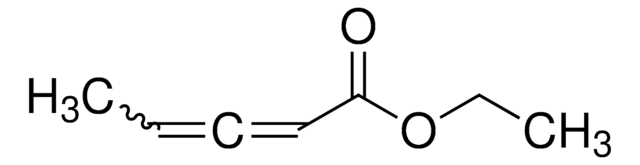
Ethyl 2,3-butadienoate
95%
Synonym(s):
(Ethoxycarbonyl)allene, Ethyl allenecarboxylate, Ethyl butadienoate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2C=C=CHCO2C2H5
CAS Number:
Molecular Weight:
112.13
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.46 (lit.)
bp
80 °C/60 mmHg (lit.)
density
0.966 g/mL at 25 °C (lit.)
functional group
ester
SMILES string
[H]C([H])=C=C([H])C(=O)OCC
InChI
1S/C6H8O2/c1-3-5-6(7)8-4-2/h5H,1,4H2,2H3
InChI key
GLSUOACRAMLJIW-UHFFFAOYSA-N
Related Categories
General description
Ethyl 2,3-butadienoate is an α-allenic ester. The reaction of ethyl 2,3-butadienoate with N-tosylated imines in the presence of DABCO (1,4-diazabicyclo[2.2.2]octane) or DMAP (4-dimethylaminopyridine) forms azetidine derivatives or novel dihydropyridine derivatives respectively. The performance of bifunctional N-acyl aminophosphines to catalyze the asymmetric [3+2] cycloaddition of phenylidenemalononitrile with ethyl 2,3-butadienoate has been evaluated.
Application
Ethyl 2,3-butadienoate may be used in the synthesis of dihydropyrans by reacting with acyclic enones. It may also be used to synthesize spiranic heterocycles by reacting with heterocyclic bis-arylidene ketones via phosphine-catalyzed [3+2] annulations.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
116.6 °F - closed cup
Flash Point(C)
47 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Abnormal aza-Baylis-Hillman reaction of N-tosylated imines with ethyl 2,3-butadienoate and penta-3,4-dien-2-one.
Zhao GL, et al.
Organic Letters, 5(24), 4737-4739 (2003)
Asymmetric [3+2] Cycloadditions of Allenoates and Dual Activated Olefins Catalyzed by Simple Bifunctional N-Acyl Aminophosphines.
Xiao H, et al.
Angewandte Chemie (International Edition in English), 49(26), 4467-4470 (2010)
Development of a formal catalytic asymmetric [4+2] addition of ethyl-2,3-butadienoate with acyclic enones.
Ashtekar KD, et al.
Organic Letters, 13(21), 5732-5735 (2011)
Heterocyclic Spiranes and Dispiranes via Enantioselective Phosphine-Catalyzed [3+2] Annulations.
Duvvuru D, et al.
Advanced Synthesis & Catalysis, 354(2-3), 408-414 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service