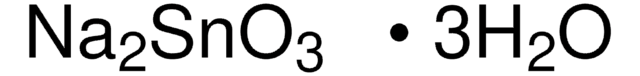379387
Dithiooxamide
97%
Synonym(s):
Dithiooxalic diamide, Rubeanic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CSCSNH2
CAS Number:
Molecular Weight:
120.20
Beilstein:
605577
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
≥300 °C (lit.)
solubility
ethanol: soluble 40 mg/10 mL, clear, red
functional group
amine
SMILES string
NC(=S)C(N)=S
InChI
1S/C2H4N2S2/c3-1(5)2(4)6/h(H2,3,5)(H2,4,6)
InChI key
OAEGRYMCJYIXQT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Dithiooxamide is reported to form complexes with Ni(II).
Application
Dithiooxamide may be used in the following studies:
- Synthesis of thiazolothiazole-linked porous organic polymers under solvothermal conditions.
- As modifier to prepare the modified glassy carbon electrode, used to investigate the electrochemical properties of quercetin, an important flavonoid derivative.
- Synthesis of new chelating resin of dithiooxamide (rubeanic acid)-formaldehyde (DTOF), used in separation and concentration of silver ions.
- Synthesis of N,N′-disubstituted dithiooxamides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xiang Zhu et al.
Chemical communications (Cambridge, England), 50(95), 15055-15058 (2014-10-21)
Thiazolothiazole-linked porous organic polymers have been synthesized from a facile catalyst-free condensation reaction between aldehydes and dithiooxamide under solvothermal conditions. The resultant porous frameworks exhibit a highly selective uptake of CO2 over N2 under ambient conditions.
Nickel (II) complexes with dithiooxamide, N, N'-di-methyl-and N, N'-di-hydroxyethyl-dithiooxamide.
Peyronel G, et al.
Inorgorganica Chimica Acta, 5, 627-633 (1971)
Preparation of Dithiooxamide Derivatives.
Hurd RN, et al.
The Journal of Organic Chemistry, 26(10), 3980-3987 (1961)
Ayşen Demir Mülazımoğlu et al.
Sensors (Basel, Switzerland), 12(4), 3916-3928 (2012-06-06)
Electrochemical oxidation of quercetin, as an important biological molecule, has been studied in non-aqueous media using cyclic voltammetry, electrochemical impedance spectroscopy and scanning electron microscopy. To investigate the electrochemical properties of quercetin, an important flavonoid derivative, on a different surface
Yurii A Simonov et al.
Organic & biomolecular chemistry, 1(16), 2922-2929 (2003-09-13)
The hydrogen-bonding networks for seven new binary compounds of dithiooxamide, (NH2CS)2 (dtox) and dithiobiurea (NH2CSNH)2 (dtur) with crown ethers, 18-crown-6 (18C6), 15-crown-5 (15C5), 12-crown-4 (12C4), cis-syn-cis-(DCHA), and cis-anti-cis-(DCHB) isomers of dicyclohexyl-18 -crown-6 are discussed. (15C5.dtox), (18C6.dtur) and (DCHB.dtur) afford one-dimensional
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service