All Photos(1)
About This Item
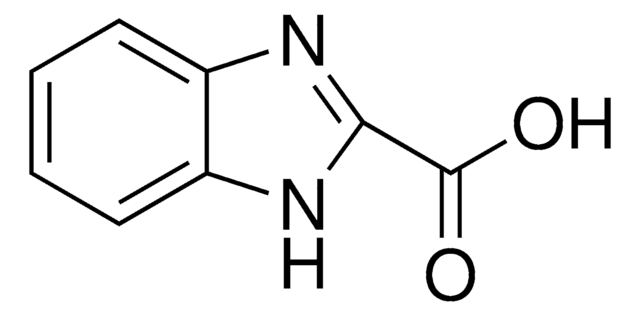
Empirical Formula (Hill Notation):
C8H6N2O2
CAS Number:
Molecular Weight:
162.15
MDL number:
UNSPSC Code:
12352100
eCl@ss:
32151902
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
mp
>300 °C (lit.)
SMILES string
OC(=O)c1ccc2[nH]cnc2c1
InChI
1S/C8H6N2O2/c11-8(12)5-1-2-6-7(3-5)10-4-9-6/h1-4H,(H,9,10)(H,11,12)
InChI key
COYPLDIXZODDDL-UHFFFAOYSA-N
General description
Drug-specific monoclonal antibodies were produced against the very small drug hapten, 5-benzimidazolecarboxylic acid.
Application
5-Benzimidazolecarboxylic acid has been used in the preparation of:
- 1H-benzoimidazole-5-carboxylic acid benzotriazol-1-yl ester
- piperidin-1-yl(1-m-tolyl-1H-benzo[d]imidazol-5-yl)methanone
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E S Medlock et al.
The Anatomical record, 207(1), 31-41 (1983-09-01)
Mouse fetal liver was studied ultrastructurally to identify and characterize the developing hepatic parenchyma or prehepatocyte which may be responsible for producing the liver hemopoietic environment. It was observed that as the liver develops, there is close association of endodermal
Ajit Jadhav et al.
Probe Reports from the NIH Molecular Libraries Program, 2010 Mar 4 (Updated 2011 Mar 11) (2011-07-08)
15-hydroxyprostaglandin dehydrogenase (15-PGDH; HPGD) is the key enzyme for the inactivation of prostaglandins, and thus regulates processes such as inflammation or proliferation. The anabolic pathways of prostaglandins are well-characterized, especially with respect to regulation of the cyclooxygenase (COX) enzymes. In
E Moran et al.
Journal of immunological methods, 271(1-2), 65-75 (2002-11-26)
Drug-specific monoclonal antibodies (MAbs) were produced against the very small drug hapten (162.15 Da), 5-benzimidazolecarboxylic acid, an analogue of 2-(4-Thiazolyl)benzimidazole (TBZ) but lacking the thiol group. TBZ is widely used as a broad-spectrum anthelmintic in various animal species and humans
Xiaofei Chen et al.
Biomaterials science, 3(6), 870-878 (2015-07-30)
Herein, hyperbranched poly(ethylene glycol)-based supramolecular nanoparticles with pH-sensitive properties were designed and used for targeted drug delivery. Via host-guest recognition between benzimidazole anchored poly(ethylene glycol)-hyperbranched polyglycerol (PEG-HPG-BM) and folic acid modified CD (FA-CD), targeted supramolecular nanoparticles (TSNs) were fabricated. At
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service