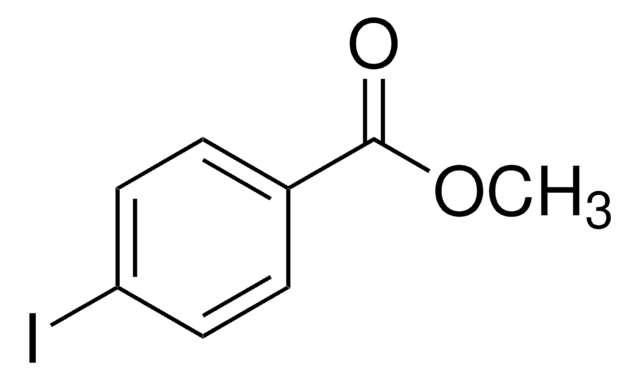
238139
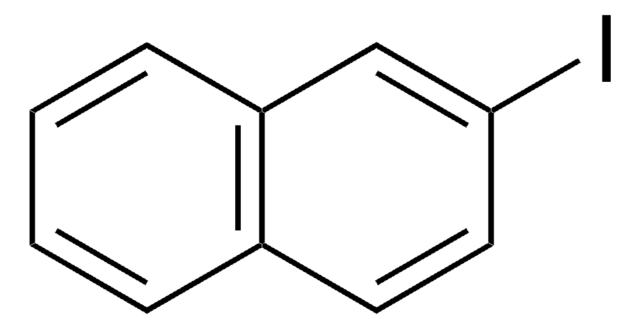
1-Iodonaphthalene
97%
Synonym(s):
1-Naphthyl iodide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H7I
CAS Number:
Molecular Weight:
254.07
Beilstein:
1906415
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.701 (lit.)
bp
163-165 °C/15 mmHg (lit.)
density
1.74 g/mL at 25 °C (lit.)
functional group
iodo
SMILES string
Ic1cccc2ccccc12
InChI
1S/C10H7I/c11-10-7-3-5-8-4-1-2-6-9(8)10/h1-7H
InChI key
NHPPIJMARIVBGU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ultrafast relaxation of 1-iodonaphthalene has been studied by time-resolved femtosecond pump-probe mass spectrometry. 1-Iodonaphthalene undergoes Pd catalyzed cross-coupling reaction (Stille reaction) with 2,4-dimethoxy-6-(trimethylstannyl)pyrimidine to afford 2,4-dimethoxy-6-(naphthalen-1-yl)pyrimidine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Javier I Bardagí et al.
The Journal of organic chemistry, 73(12), 4491-4495 (2008-05-21)
From the commercial 6-chloro-2,4-dimethoxypyrimidine (1) and by a photostimulated reaction with Me(3)Sn(-) ions, 2,4-dimethoxy-6-(trimethylstannyl)pyrimidine (2) was obtained in 95% yield. By the cross-coupling reaction of 2 with 1-iodonaphthalene as electrophile catalyzed by Pd (Stille reaction), 2,4-dimethoxy-6-(naphthalen-1-yl)pyrimidine (9) was obtained in
Raul Montero et al.
Physical chemistry chemical physics : PCCP, 12(28), 7988-7993 (2010-06-03)
The ultrafast relaxation of 1-iodonaphthalene, with particular attention to the dissociation channels, has been studied by time-resolved femtosecond pump-probe mass spectrometry following excitation at 267 and 317 nm. The measured transients for the parent ion and the isobaric fragments, iodine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service