118192
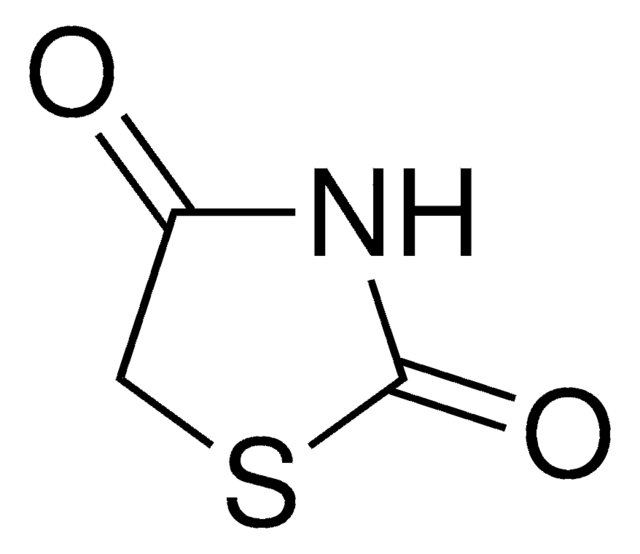
Rhodanine
97%
Synonym(s):
2-Thioxo-4-thiazolidinone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C3H3NOS2
CAS Number:
Molecular Weight:
133.19
Beilstein:
110701
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
165-169 °C (lit.)
solubility
methanol: soluble 2.5%, clear (yellow-green to orange-brown)
SMILES string
O=C1CSC(=S)N1
InChI
1S/C3H3NOS2/c5-2-1-7-3(6)4-2/h1H2,(H,4,5,6)
InChI key
KIWUVOGUEXMXSV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Rhodanine possess anticonvulsant, antibacterial, antiviral and antidiabetic activities.
Application
Rhodanine has been used in tannase assay in cultures of tannin degrading fungi.
Biochem/physiol Actions
Rhodanine inhibits the multiplication of echovirus 12 and the development of virus-induced morphologic changes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Neil S Cutshall et al.
Bioorganic & medicinal chemistry letters, 15(14), 3374-3379 (2005-06-18)
Dual-specificity phosphatases (DSPs) are a subclass within the protein tyrosine phosphatase family (PTPs). A series of rhodanine-based inhibitors was synthesized and shown to be novel, potent, and selective inhibitors against the DSP family member JNK-stimulating phosphatase-1 (JSP-1). Compounds of this
N Hotta et al.
Diabetic medicine : a journal of the British Diabetic Association, 29(12), 1529-1533 (2012-04-18)
The goal of the study was to evaluate the efficacy of epalrestat, an aldose reductase inhibitor, on diabetic retinopathy and diabetic nephropathy, based on analysis of the results of the Aldose Reductase Inhibitor-Diabetes Complications Trial, a 3-year multicentre comparative clinical
Thomas Mendgen et al.
Journal of medicinal chemistry, 55(2), 743-753 (2011-11-15)
Rhodanines and related five-membered heterocycles with multiple heteroatoms have recently gained a reputation of being unselective compounds that appear as "frequent hitters" in screening campaigns and therefore have little value in drug discovery. However, this judgment appears to be based
Feng Yu et al.
Organic letters, 14(8), 2038-2041 (2012-04-10)
A bulky group was introduced by design into a diamine catalyst, and a series of robust and tunable bulky chiral primary amine catalysts were developed and successfully applied in the direct conjugate addition of substituted rhodanines to α,β-unsaturated ketones. High
T Tomasić et al.
Current medicinal chemistry, 16(13), 1596-1629 (2009-05-16)
Rhodanines, thiazolidine-2,4-diones and pseudothiohydantoins have become a very interesting class of heterocyclic compounds since the introduction of various glitazones and epalrestat into clinical use for the treatment of type II diabetes mellitus and diabetic complications, respectively. Chemical modifications of these
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service