S1826
Sialic Acid Aldolase from Escherichia coli K12
recombinant, expressed in E. coli BL21, ≥3.0 units/mg protein
Synonym(s):
N-Acetylneuraminate lyase, N-Acetylneuraminate pyruvate-lyase (N-acetyl-D-mannosamine-forming)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in E. coli BL21
Quality Level
form
lyophilized powder
specific activity
≥3.0 units/mg protein
mol wt
33.4 kDa
shipped in
dry ice
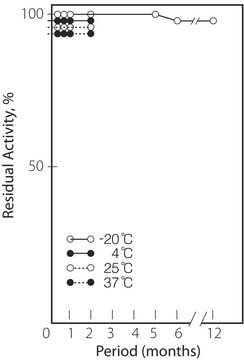
storage temp.
−20°C
General description
Sialic acid aldolases, or N-acetylneuraminate lyases, catalyze the reversible aldol cleavage of N-acetylneuraminic acid to form pyruvate and N-acetyl-D-mannosamine. In nature, N-acetylneuraminate lyase mainly occurs in pathogens.
Application
Sialic acid aldolase can be used to synthesize unnatural sugars of C(6) to C(10) for the design of antagonists and inhibitors of glycoenzymes.
Unit Definition
One unit will catalyze the formation of 1.0 μmol Neu-5-Ac from Man-N-Ac and pyruvate per minute at 37°C at pH 8.0.
Physical form
Lyophilized powder containing Tris-HCl and NaCl
Analysis Note
Enzymatic activity assays are performed in Tris-HCl buffer (100 mM, pH 7.5) containing Neu-5-Ac (10 mM) at 37 °C for 15 min and analyzed using capillary electrophoresis with UV detection at 200 nm.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Guiomar Sánchez-Carrón et al.
Applied and environmental microbiology, 77(7), 2471-2478 (2011-02-15)
N-Acetylneuraminate lyases (NALs) or sialic acid aldolases catalyze the reversible aldol cleavage of N-acetylneuraminic acid (Neu5Ac) to form pyruvate and N-acetyl-d-mannosamine (ManNAc). In nature, N-acetylneuraminate lyase occurs mainly in pathogens. However, this paper describes how an N-acetylneuraminate lyase was cloned
Modulation of substrate specificities of D-sialic acid aldolase through single mutations of Val-251.
Chien-Yu Chou et al.
The Journal of biological chemistry, 286(16), 14057-14064 (2011-01-29)
In a recent directed-evolution study, Escherichia coli D-sialic acid aldolase was converted by introducing eight point mutations into a new enzyme with relaxed specificity, denoted RS-aldolase (also known formerly as L-3-deoxy-manno-2-octulosonic acid (L-KDO) aldolase), which showed a preferred selectivity toward
Jozef Nahálka et al.
Organic & biomolecular chemistry, 7(9), 1778-1780 (2009-07-11)
Active inclusion bodies of polyphosphate kinase 3 and cytidine 5'-monophosphate kinase were combined with whole cells that co-express sialic acid aldolase and CMP-sialic acid synthetase. The biocatalytic mixture was used for the synthesis of CMP-sialic acid, which was then converted
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service