All Photos(1)
About This Item
Linear Formula:
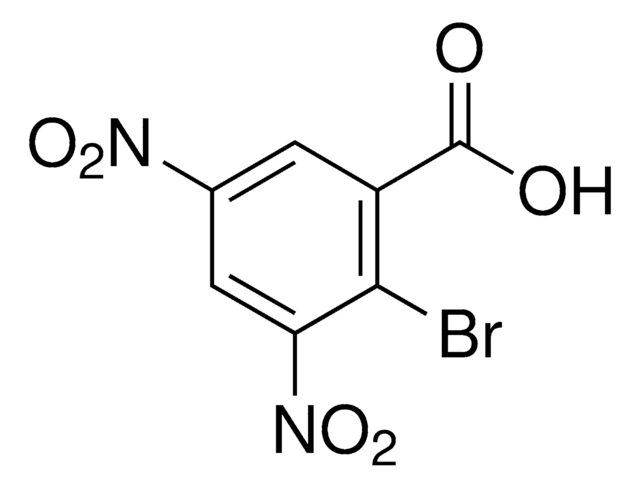
CH3OC6H3(NO2)CO2H
CAS Number:
Molecular Weight:
197.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
146-148 °C (lit.)
functional group
carboxylic acid
nitro
SMILES string
COc1cc(ccc1C(O)=O)[N+]([O-])=O
InChI
1S/C8H7NO5/c1-14-7-4-5(9(12)13)2-3-6(7)8(10)11/h2-4H,1H3,(H,10,11)
InChI key
KPJXEWJRJKEOCD-UHFFFAOYSA-N
Related Categories
General description
2-Methoxy-4-nitrobenzoic acid is an alkoxybenzoic acid derivative. It has been reported to be synthesized by reacting 2-hydroxy-4-nitrobenzoic acid with methyl iodide and characterized by 1H and 13C-NMR spectra.
Application
2-Methoxy-4-nitrobenzoic acid may be used as a starting material in the following syntheses:
- 2-Methoxy-4-nitrobenzamide, a nitroamide derivative.
- 4-Amino-2-methoxybenzamide, an aminobenzamide derivative.
- N,2-Dimethoxy-N-methyl-4-nitrobenzamide, a Weinreb amide derivative.
- 1-(2-Methoxy-4-nitrophenyl)-5-methylhex-2-yn-1-one, a ynone derivative.
- 2,5-Constrained piperidine derivative, a potential CCR3 (Chemokine (C-C Motif) Receptor 3) antagonist.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Leyi Gong et al.
Bioorganic & medicinal chemistry letters, 13(20), 3597-3600 (2003-09-25)
As part of our investigation into the development of potent CCR3 antagonists, a series of piperidine analogues was designed and prepared. Exploration of the piperidine core examined both the basicity and the location of a nitrogen, as well as conformational
Marc J Adler et al.
The Journal of organic chemistry, 76(17), 7040-7047 (2011-07-12)
The design and synthesis of small molecule α-helix mimetics has been a productive field over the past decade. These compounds have performed well in a variety of biological systems as functional disruptors of α-helix-mediated protein-protein interactions. In our studies we
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service