332593
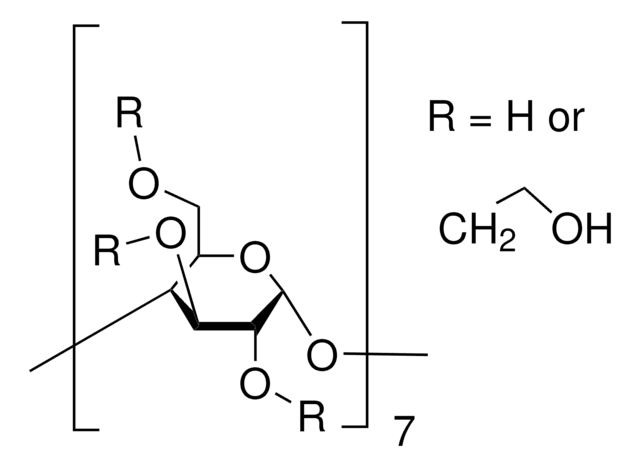
(2-Hydroxypropyl)-β-cyclodextrin
average Mw ~1,380
Synonym(s):
(2-Hydroxypropyl)-beta-cyclodextrin
About This Item
Recommended Products
form
powder
optical activity
[α]26/D +139°, c = 1 in H2O
mol wt
average Mw ~1,380
extent of labeling
0.6 molar substitution
mp
278 °C (dec.)
SMILES string
CC(O)COCC1OC2OC3C(COCC(C)O)OC(OC4C(COCC(C)O)OC(OC5C(COCC(C)O)OC(OC6C(COCC(C)O)OC(OC7C(COCC(C)O)OC(OC8C(COCC(C)O)OC(OC1C(OCC(C)O)C2OCC(C)O)C(OCC(C)O)C8OCC(C)O)C(OCC(C)O)C7OCC(C)O)C(OCC(C)O)C6OCC(C)O)C(OCC(C)O)C5OCC(C)O)C(OCC(C)O)C4OCC(C)O)C(OCC(C)O)C3OCC(C)O
InChI
1S/C63H112O42/c1-22(64)8-85-15-29-50-36(71)43(78)57(92-29)100-51-30(16-86-9-23(2)65)94-59(45(80)38(51)73)102-53-32(18-88-11-25(4)67)96-61(47(82)40(53)75)104-55-34(20-90-13-27(6)69)98-63(49(84)42(55)77)105-56-35(21-91-14-28(7)70)97-62(48(83)41(56)76)103-54-33(19-89-12-26(5)68)95-60(46(81)39(54)74)101-52-31(17-87-10-24(3)66)93-58(99-50)44(79)37(52)72/h22-84H,8-21H2,1-7H3/t22?,23?,24?,25?,26?,27?,28?,29-,30-,31?,32?,33?,34?,35?,36?,37-,38?,39-,40+,41+,42+,43?,44+,45?,46+,47+,48+,49+,50+,51+,52-,53-,54-,55-,56-,57+,58-,59+,60-,61-,62-,63-/m1/s1
InChI key
ODLHGICHYURWBS-RYJYQAAZSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As an additive in the separation of amino acids and lactic acids based on the coordination interactions between Cu2+ ions and analytes.
- As a chiral selector in capillary electrophoresis, enabling the robust separation of diastereomeric derivatives of aspartic acid for analysis.
- As a reagent to improve water solubility and anti-proliferative activity of pyrazolo[3,4-d] pyrimidines.
- As formulation vehicle. It increases water solubility of the drug for successful delivery of medical agents to a biological system.
- To increase the solubility of ropivacaine (RVC) upon complexation, which in turn enhances the pharmacological activity of RVC.
- To increase the aqueous solubility of naproxen (NAP) in presence of polyvinylpyrrolidone (PVP).
- As a template in the synthesis of hollow spherical copper sulfide nanoparticle assemblies.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
>752.0 °F
Flash Point(C)
> 400 °C
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service