M0285
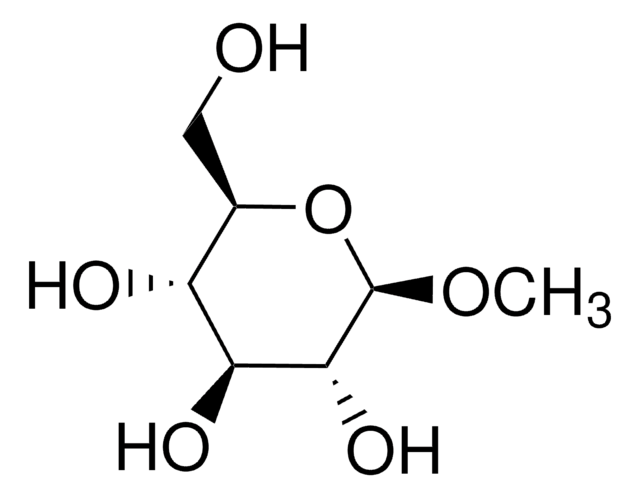
Methyl-β-D-galactopyranoside
Synonym(s):
Methyl β-D-galactoside
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H14O6
CAS Number:
Molecular Weight:
194.18
Beilstein:
81569
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
biological source
synthetic
Quality Level
Assay
≥98% (GC)
form
powder
technique(s)
gas chromatography (GC): suitable
color
white to faint yellow
mp
176-179 °C
solubility
water: 50 mg/mL, clear, colorless
storage temp.
2-8°C
SMILES string
CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O
InChI
1S/C7H14O6/c1-12-7-6(11)5(10)4(9)3(2-8)13-7/h3-11H,2H2,1H3/t3-,4+,5+,6-,7-/m1/s1
InChI key
HOVAGTYPODGVJG-VOQCIKJUSA-N
Looking for similar products? Visit Product Comparison Guide
General description
A β-D-galactopyranoside having a methyl substituent at the anomeric position.
Biochem/physiol Actions
Methyl galactoside is a hexose involved in the metabolism of 2-deoxygalactose.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Mayer et al.
Journal of the American Chemical Society, 123(25), 6108-6117 (2001-06-21)
A protocol based on saturation transfer difference (STD) NMR spectra was developed to characterize the binding interactions at an atom level, termed group epitope mapping (GEM). As an example we chose the well-studied system of galactose binding to the 120-kDa
Z A Popper et al.
Phytochemistry, 57(5), 711-719 (2001-06-09)
Acid hydrolysis of cell wall-rich material from young leaves of the lycophyte Selaginella apoda (L.) Spring yielded substantial amounts of 3-O-methyl-D-galactose (1) in addition to the usual major monosaccharides (glucose, galactose, arabinose, xylose and galacturonic acid). The yield of 1
J Aqvist et al.
The Journal of biological chemistry, 270(17), 9978-9981 (1995-04-28)
A new theoretical method for free energy calculations is used to compute the absolute binding constants for beta-D-glucose and methyl-beta-D-galactoside to the periplasmic glucose/galactose receptor from Salmonella typhimurium. The computer simulation results agree well with available experimental data and make
T J Kelley et al.
Bioscience reports, 19(5), 433-447 (2000-04-14)
The highly purified DNA Pol-alpha from rat prostate tumor (PA-3) and human neuroblastoma (IMR-32) cells appeared to be inhibited by Ricin (RCA-II), and Con-A. Loss of activity (40 to 60%) of a specific form of DNA polymerase from IMR-32 was
Peter Capek
Carbohydrate research, 343(8), 1390-1393 (2008-04-25)
An arabinogalactan with a high content of 3-O-methyl-D-galactose residues has been isolated from the aerial parts of sage (Salvia officinalis L.). Structural studies of the polymer indicated a beta-1,6-D-galactopyranose backbone in which at least one-third of D-galactopyranosyl residues carries methoxyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service