V209
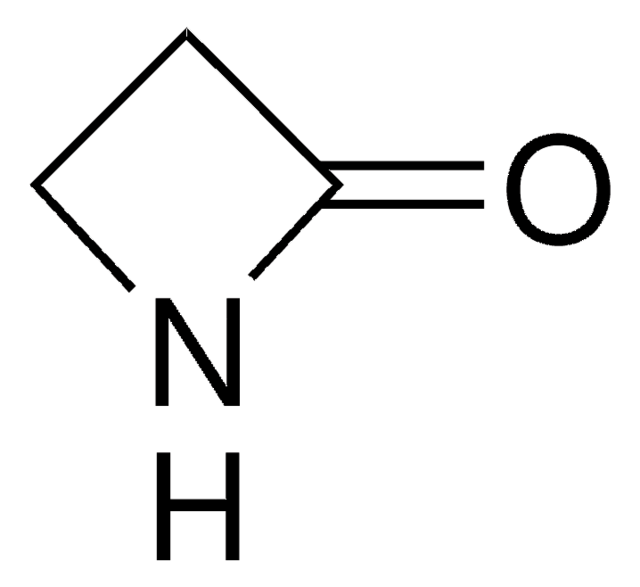
δ-Valerolactam
98%
Synonym(s):
delta-Valerolactam, 2-Piperidone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H9NO
CAS Number:
Molecular Weight:
99.13
Beilstein:
106434
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
256 °C (lit.)
81-82 °C/0.1 mmHg (lit.)
mp
38-40 °C (lit.)
SMILES string
O=C1CCCCN1
InChI
1S/C5H9NO/c7-5-3-1-2-4-6-5/h1-4H2,(H,6,7)
InChI key
XUWHAWMETYGRKB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Haili Lin et al.
Experimental & molecular medicine, 52(3), 367-379 (2020-03-11)
The function of the fibrinolytic system was first identified to dissolve fibrin to maintain vascular patency. Connections between the fibrinolytic system and many other physiological and pathological processes have been well established. Dysregulation of the fibrinolytic system is closely associated
Ahmed Mahjoub et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 12(10), 1822-1832 (2011-05-28)
We studied the single-photon ionization of gas-phase δ-valerolactam (piperidin-2-one) and of its dimer using vacuum-ultraviolet (VUV) synchrotron radiation coupled to a velocity map imaging electron/ion coincidence spectrometer. The slow photoelectron spectrum (SPES) of the monomer is dominated by the vibrational
Moitrayee Mukherjee et al.
The journal of physical chemistry. A, 116(40), 9888-9896 (2012-09-19)
A comparative analysis for relative stability between normal and tautomeric forms in the excited electronic states of 7-azaindole···δ-valerolactam 1:1 complex and 7-azaindole homodimer has been presented. The tautomeric configuration of the complex is estimated to be ~6 kcal/mol more stable
Metabolic engineering of Escherichia coli for the production of four-, five- and six-carbon lactams.
Tong Un Chae et al.
Metabolic engineering, 41, 82-91 (2017-04-10)
Microbial production of chemicals and materials from renewable sources is becoming increasingly important for sustainable chemical industry. Here, we report construction of a new and efficient platform metabolic pathway for the production of four-carbon (butyrolactam), five-carbon (valerolactam) and six-carbon (caprolactam)
Mitchell G Thompson et al.
ACS synthetic biology, 9(1), 53-62 (2019-12-17)
Caprolactam is an important polymer precursor to nylon traditionally derived from petroleum and produced on a scale of 5 million tons per year. Current biological pathways for the production of caprolactam are inefficient with titers not exceeding 2 mg/L, necessitating
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service