All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C4H6N4
CAS Number:
Molecular Weight:
110.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
143-147 °C (lit.)
SMILES string
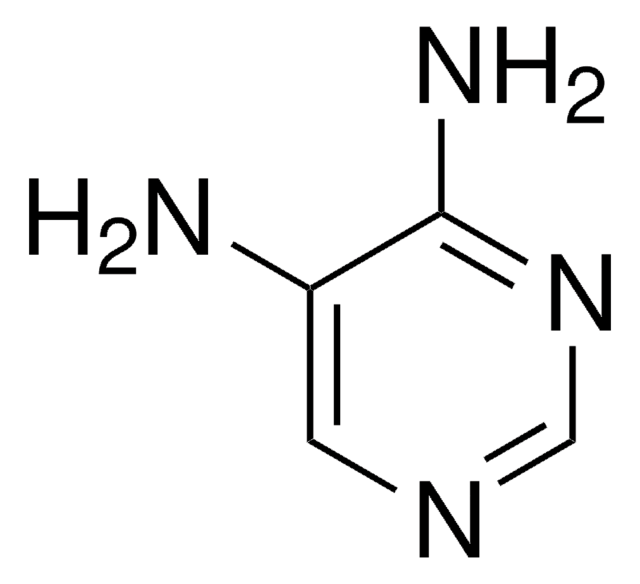
Nc1ccnc(N)n1
InChI
1S/C4H6N4/c5-3-1-2-7-4(6)8-3/h1-2H,(H4,5,6,7,8)
InChI key
YAAWASYJIRZXSZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wilhelm Maximilian Hützler et al.
Acta crystallographica. Section C, Structural chemistry, 71(Pt 3), 229-238 (2015-03-04)
The results of seven cocrystallization experiments of the antithyroid drug 6-methyl-2-thiouracil (MTU), C(5)H(6)N(2)OS, with 2,4-diaminopyrimidine, 2,4,6-triaminopyrimidine and 6-amino-3H-isocytosine (viz. 2,6-diamino-3H-pyrimidin-4-one) are reported. MTU features an ADA (A = acceptor and D = donor) hydrogen-bonding site, while the three coformers show
Wenbo Zhou et al.
European journal of medicinal chemistry, 96, 269-280 (2015-04-23)
Therapeutics of metastatic or triple-negative breast cancer are still challenging in clinical. Herein we demonstrated the design and optimization of a series of hybrid of 2,4-diaminopyrimidine and arylthiazole derivatives for their anti-proliferative properties against two breast cancer cell lines (MCF-7
Mary J Carroll et al.
Nature chemical biology, 8(3), 246-252 (2012-01-17)
Signal transduction, regulatory processes and pharmaceutical responses are highly dependent upon ligand residence times. Gaining insight into how physical factors influence residence times (1/k(off)) should enhance our ability to manipulate biological interactions. We report experiments that yield structural insight into
Baskar Nammalwar et al.
European journal of medicinal chemistry, 54, 387-396 (2012-06-19)
A series of substituted 2,4-diaminopyrimidines 1 has been prepared and evaluated for activity against Bacillus anthracis using previously reported (±)-3-{5-[(2,4-diamino-5-pyrimidinyl)methyl]-2,3-dimethoxyphenyl}-1-(1-propyl-2(1H)-phthalazinyl)-2-propen-1-one (1a), with a minimum inhibitory concentration (MIC) value of 1-3 μg/mL, as the standard. In the current work, the corresponding
Eugen F Mesaros et al.
Bioorganic & medicinal chemistry letters, 21(1), 463-466 (2010-11-16)
The synthesis and biological evaluation of potent and selective anaplastic lymphoma kinase (ALK) inhibitors from a novel class of 2,4-diaminopyrimidines, incorporating 2,3,4,5-tetrahydro-benzo[d]azepine fragments, is described. An orally bioavailable analogue (18) that displayed antitumor efficacy in ALCL xenograft models in mice
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service