1205003
USP
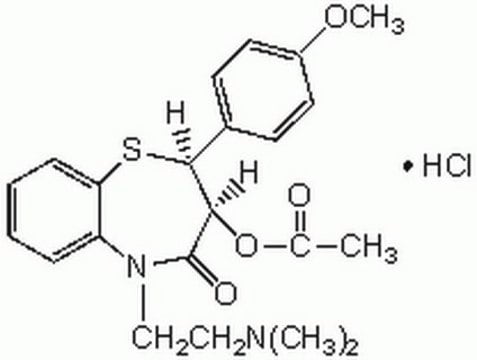
Diltiazem hydrochloride
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
(+)-cis-Diltiazem hydrochloride, (2S,3S)-(+)-cis-3-Acetoxy-5-(2-dimethylaminoethyl)-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one hydrochloride, CRD-401
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
diltiazem
manufacturer/tradename
USP
application(s)
pharmaceutical (small molecule)
format
neat
SMILES string
Cl.COc1ccc(cc1)[C@@H]2Sc3ccccc3N(CCN(C)C)C(=O)[C@@H]2OC(C)=O
InChI
1S/C22H26N2O4S.ClH/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3;/h5-12,20-21H,13-14H2,1-4H3;1H/t20-,21+;/m1./s1
InChI key
HDRXZJPWHTXQRI-BHDTVMLSSA-N
Gene Information
human ... CACNA1C(775) , CACNA1D(776) , CACNA1F(778) , CACNA1S(779)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Exploring the Effectiveness of Carboxymethylated and Crosslinked Albizia procera Gum in Diltiazem Hydrochloride Matrix Tablets: A Comparative Analysis. This study investigates the potential of carboxymethylated and crosslinked Albizia procera gum for use in sustained-release matrix tablets containing Diltiazem hydrochloride, highlighting its viability in pharmaceutical applications (Mukherjee S, Khanam J, 2024).
- Dietary gallic acid as an antioxidant: A review of its food industry applications, health benefits, bioavailability, nano-delivery systems, and drug interactions. While primarily focused on gallic acid, this review mentions Diltiazem hydrochloride in the context of drug interactions and its implications in food safety and health (Xiang Z et al., 2024).
- Dual stimuli-responsive and sustained drug delivery NanoSensoGel formulation for prevention of cisplatin-induced ototoxicity. This research presents a novel NanoSensoGel that could be adapted for Diltiazem hydrochloride, enhancing drug delivery efficiency in clinical settings (Thakur NS et al., 2024).
- A novel potentiometric sensor based on ZnO decorated polyaniline/coal nanocomposite for diltiazem determination. This study develops a new sensor for the precise measurement of Diltiazem levels in pharmaceutical formulations, which is crucial for quality control and regulatory compliance (El Sayed GA et al., 2023).
- The Effect of Topical Nifedipine versus Diltiazem on the Acute Anal Fissure: A Randomized Clinical Trial. This clinical trial evaluates the effectiveness of Diltiazem hydrochloride in treating acute anal fissures, providing evidence of its beneficial applications in proctological disorders (Momayez Sanat Z et al., 2023).
Analysis Note
Other Notes
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service