SAE0021
Pyruvate Kinase M2 human
recombinant, expressed in E. coli, specific activity ≥100 unit/mg protein
Synonym(s):
Cytosolic Thyroid hormone-binding protein (CTHBP), M2-PK, OPA-interacting protein 3 (OIP-3), PKM2, Pyruvate kinase 3 (PK3), Pyruvate kinase muscle isozyme, p58
Sign Into View Organizational & Contract Pricing
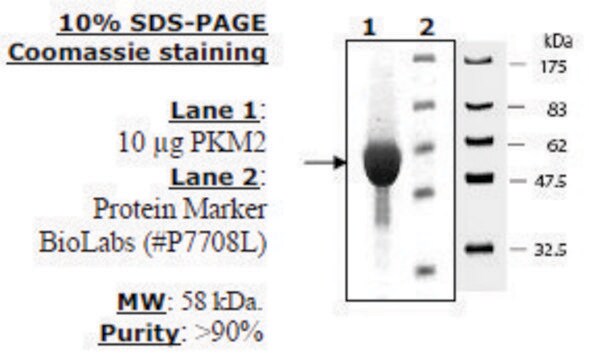
All Photos(1)
About This Item
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in E. coli
Quality Level
form
lyophilized powder
specific activity
≥100 units/mg protein
shipped in
ambient
storage temp.
−20°C
General description
Research area: Cell signaling This product is recombinant human PKM2 (P14618) expressed in E. coli and has a predicted molecular mass of 58 kDa. The embryonic pyruvate kinase M2 (PKM2) is one of the isoforms of pyruvate kinases (PK), found in mammals. It is coded by the PKM2 gene mapped to human chromosome 15q23. PKM2 is expressed in all cells excluding adult muscle, brain, and liver. This protein is usually present, as an active tetrameric form.
Application
Human pyruvate kinase M2 (PKM2) has been used to study the effects of phosphorylated PKM2 in inactivating myofibroblasts during renal fibrosis.PyruvateKinase M2 human has been used as an enzyme to study the inhibitory effect of a novel irreversible inhibitor on the kinaseactivity of PKM2. It has also been used in recombinant protein labelingand in pyruvate kinase enzyme activity assay.
Biochem/physiol Actions
Pyruvate kinase (PK) is an important glycolytic enzyme catalyzing the last step of glycolysis, transferring a phosphate group from phosphoenolpyruvate to ADP to yield one molecule of ATP and one molecule of pyruvate. The embryonic pyruvate kinase M2 (PKM2) controls β-catenin transactivation. It plays an important role in aerobic glycolysis or the Warburg effect. PKM2 induces gene transcription and tumorigenesis.Pyruvate Kinase M2 (PKM2)plays a role in the regulation of cell growth, cell proliferation, andapoptosis. It is found predominantly in embryonic tissues and several humancancers. PKM2 acts as a cytosolic thyroid hormone binding protein(CTHBP) and as a phosphotyrosine binding protein. PKM2 binds tophosphotyrosine peptides which results in the release of its allostericactivator fructose-1,6-bisphosphate (FBP) is associated with the blockageof PKM2 activity.
Unit Definition
One unit will convert 1.0 μmole of phosphoenol-pyruvate to pyruvate per minute at pH 7.6 at 37 °C, in the presence of 1 mM fructose-1,6-bisphosphate.
Physical form
Supplied as a lyophilized powder containing phosphate buffer at pH7.5, NaCl, DTT and a carbohydrate stabilizer.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
I-Shan Hsieh et al.
Journal of medicinal chemistry, 62(18), 8497-8510 (2019-08-30)
As cancer cells undergo metabolic reprogramming in the course of tumorigenesis, targeting energy metabolism represents a promising strategy in cancer therapy. Among various metabolic enzymes examined, pyruvate kinase M2 type (PKM2) has received much attention in light of its multifaceted
Yeonjin Ko et al.
Proceedings of the National Academy of Sciences of the United States of America, 120(20), e2300763120-e2300763120 (2023-05-08)
KEAP1 (Kelch-like ECH-associated protein), a cytoplasmic repressor of the oxidative stress responsive transcription factor Nuclear factor erythroid 2-related factor 2 (NRF2), senses the presence of electrophilic agents by modification of its sensor cysteine residues. In addition to xenobiotics, several reactive
Inhibiting aerobic glycolysis suppresses renal interstitial fibroblast activation and renal fibrosis
Ding H, et al.
American Journal of Physiology: Renal Physiology, 313(3), F561-F575 (2017)
Pyruvate kinase isoenzyme M2 expression correlates with survival of cardiomyocytes after allogeneic rat heterotopic heart transplantation.
Shi J, et al.
Pathology Research and Practice, 211(1), 12-19 (2015)
Sagarkumar Patel et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 170, 106112-106112 (2022-01-01)
Discovery of novel and potent lead molecules for the specific therapeutic targets by de novo drug design is still in infancy. Here, we disclose the unprecedented development of imidazopyri(mi)dine-based tumor pyruvate kinase M2 (PKM2) modulators by subsequent link and grow strategy. The most potent
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service