T2005010
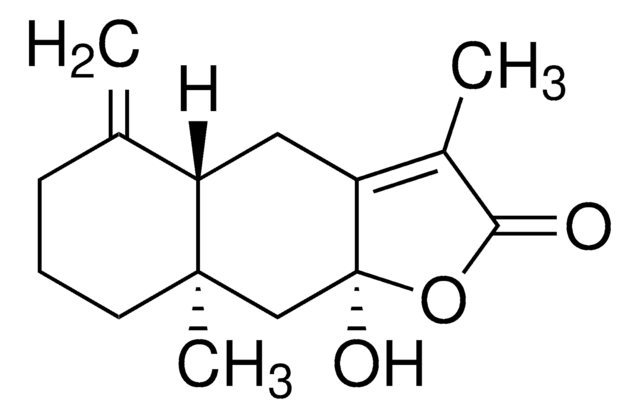
Triflusal impurity B
European Pharmacopoeia (EP) Reference Standard
Synonym(s):
2-Hydroxy-4-(trifluoromethyl)benzoic acid, 4-(Trifluoromethyl)salicylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H5F3O3
CAS Number:
Molecular Weight:
206.12
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
API family
triflusal
manufacturer/tradename
EDQM
application(s)
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
InChI
1S/C8H5F3O3/c9-8(10,11)4-1-2-5(7(13)14)6(12)3-4/h1-3,12H,(H,13,14)
InChI key
XMLFPUBZFSJWCN-UHFFFAOYSA-N
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Triflusal impurity B EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J A González-Correa et al.
Naunyn-Schmiedeberg's archives of pharmacology, 371(1), 81-88 (2004-12-17)
Triflusal is a fluorinated derivative of acetylsalicylic acid (ASA) with demonstrated antithrombotic activity. Recently, evidence for a neuroprotective effect has been obtained. The aim of this study was to compare the neuroprotective effects of the main metabolite of triflusal (2-hydroxy-4-trifluoromethylbenzoic
Anna Argemí et al.
Journal of pharmaceutical and biomedical analysis, 46(3), 456-462 (2007-12-21)
This study describes the development and evaluation of an analytical method for the characterization of triflusal (2-acetoxy-4-(trifluoromethyl) benzoic acid) dispersed in sustained delivery systems prepared using supercritical fluid impregnation technology. Characterization assays comprised the determination of the percentage of triflusal
C Velasco et al.
The Journal of urology, 166(5), 1962-1968 (2001-10-05)
We examined the effects of intravenous administration of the 2 nuclear factor-kappaB inhibitors aspirin and 2-hydroxy-4-trifluoromethylbenzoic acid (HTB) on bladder filling and voiding in anesthetized and conscious rats. Disappearance of isovolumic bladder contractions after intravenous administration of different doses of
A Fernández de Arriba et al.
Molecular pharmacology, 55(4), 753-760 (1999-04-01)
The therapeutic potential of drugs that block the induction of cyclooxygenase-2 has been emphasized. When two 4-trifluoromethyl salicylate derivatives [2-acetoxy-4-trifluoromethyl-benzoic acid (triflusal) and its deacetylated metabolite 2-hydroxy-4-trifluoromethylbenzoic acid (HTB)] were compared with aspirin and sodium salicylate as cyclooxygenase-2 (COX-2) inhibitors
Y Bayón et al.
British journal of pharmacology, 126(6), 1359-1366 (1999-04-27)
1. The effect of two derivatives of salicylate, 2-hydroxy-4-trifluoromethylbenzoic acid (HTB) and 2-acetoxy-4-trifluoromethylbenzoic acid (triflusal), on the activation of NF-kappaB elicited by tumour necrosis factor-alpha (TNF-alpha) on human umbilical vein endothelial cells (HUVEC) was tested. 2. The expression of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service