I4386
Iodoacetic acid
≥98.0% (T)
Synonym(s):
2-Iodoacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
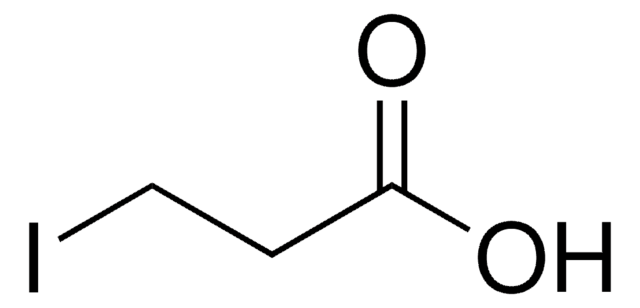
Linear Formula:
ICH2CO2H
CAS Number:
Molecular Weight:
185.95
Beilstein:
1739079
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
biological source
synthetic
Quality Level
Assay
≥98.0% (T)
form
powder or flakes
color
white to yellow
mp
77-79 °C (lit.)
solubility
H2O: soluble, clear to hazy
storage temp.
−20°C
SMILES string
OC(=O)CI
InChI
1S/C2H3IO2/c3-1-2(4)5/h1H2,(H,4,5)
InChI key
JDNTWHVOXJZDSN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Iodoacetic acid has been used as an alkylating agent for antibody AR37, serum proteins and strawberry proteome samples extracted from different stages of ripened fruit.
Reagent for the modification of cysteine residues in proteins.
Biochem/physiol Actions
Iodoacetic acid (IAA) blocks the thiol group of cysteine. IAA inhibits glyceraldehyde-3-phosphate dehydrogenase (G3PDH) by interacting with sulfhydryl group of the active site cysteine. IAA inhibits the progression of solid Ehrlich carcinoma. IAA is one of the iodinated disinfection byproducts in drinking water. It is cytotoxic to mammalian cells.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1A
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Antitumor activities of iodoacetate and dimethylsulphoxide against solid Ehrlich carcinoma growth in mice
Fahim FA, et al.
Biological Research, 36(2), 253-262 (2003)
Development of candidate biomarkers for pancreatic ductal adenocarcinoma using multiple reaction monitoring
Yu J, et al.
Biotechnology and Bioprocess Engineering: BBE, 18(5), 1038-1047 (2013)
Database-independent Protein Sequencing (DiPS) Enables Full-length de Novo Protein and Antibody Sequence Determination
Savidor A, et al.
Molecular and Cellular Proteomics, 16(6), 1151-1161 (2017)
Carboxymethylation of cysteine using iodoacetamide/iodoacetic acid
The Protein Protocols Handbook, 455-456 (2002)
Quantitative proteomic investigation employing stable isotope labeling by peptide dimethylation on proteins of strawberry fruit at different ripening stages
Li L, et al.
Journal of Proteomics, 94(5), 219-239 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service