D9805
Fast Blue B Salt
Dye content ~95 %, Powder
Synonym(s):
o-Dianisidine bis(diazotized) zinc double salt, Azoic Diazo No. 48, DBB, Diazo Blue B, Naphthanil Diazo Blue B
About This Item
Recommended Products
product name
Fast Blue B Salt, Dye content ~95 %
description
Technical grade
Quality Level
form
powder
composition
Dye content, ~95%
technique(s)
microbe id | staining: suitable
color
yellow to dark brown
mp
>300 °C (lit.)
solubility
H2O: 1 mg/mL
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
2-8°C
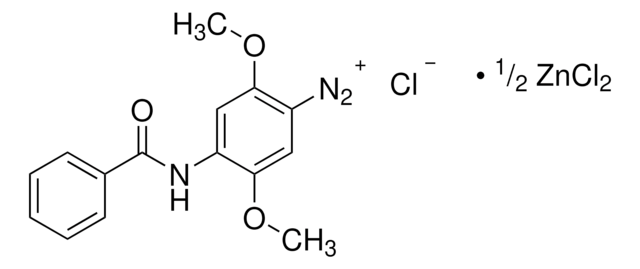
SMILES string
[Cl-].[Cl-].Cl[Zn]Cl.COc1cc(ccc1[N+]#N)-c2ccc([N+]#N)c(OC)c2
InChI
1S/C14H12N4O2.4ClH.Zn/c1-19-13-7-9(3-5-11(13)17-15)10-4-6-12(18-16)14(8-10)20-2;;;;;/h3-8H,1-2H3;4*1H;/q+2;;;;;+2/p-4
InChI key
GPPKNJIWDULNQH-UHFFFAOYSA-J
Looking for similar products? Visit Product Comparison Guide
Application
- Fast Blue B has been used in the acid phosphatase test in Clostridium perfringens.
- It has been used as a spraying agent in thin layer chromatography for the identification of separated components.
- It has also been used for the determination of the lipase activity.
Biochem/physiol Actions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 1B - Self-react. C
Storage Class Code
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service