14-440-M
PKA Protein, Recombinant, 10 µg
Recombinant human full length PKA, catalytic subunit type alpha, untagged, for use in Kinase Assays.
About This Item
Recommended Products
biological source
human
Quality Level
recombinant
expressed in E. coli
expressed in BL21 DE3 cells
product line
Upstate®
form
liquid
specific activity
(For Specific Activity data, refer to the Certificate of Analysis for individual lots of this enzyme.)
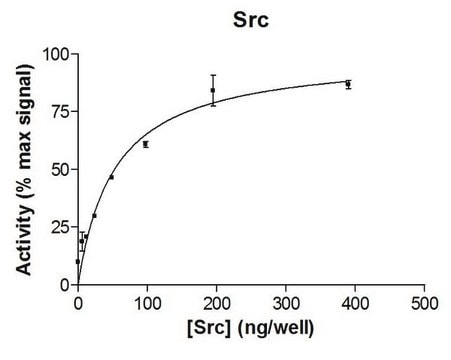
8580 U/mg
shelf life
1 yr
mol wt
Mw 40.7 kDa
purified by
chromatography
species reactivity
human
manufacturer/tradename
Upstate®
technique(s)
activity assay: suitable (kinase)
solubility
water: soluble
accession no.
(FUNCTION: SwissProt: P17612 # Phosphorylates a large number of substrates in the cytoplasm and the nucleus. )
NM_002730.3
NCBI accession no.
UniProt accession no.
storage temp.
−70°C
Gene Information
human ... PRKACA(46909581)
General description
Product Source: Expressed in E.coli BL21 (DE3) pLysS cells Recombinant human full length PKA, catalytic subunit type alpha, untagged Protein kinase A (PKA) is a serine protein kinase and heterotetrameric holoenzyme that targets serine and threonine residues.
Biochem/physiol Actions
Packaging
Quality
Target description
Linkage
Physical form
Storage and Stability
Other Notes
Legal Information
Disclaimer
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service