General description
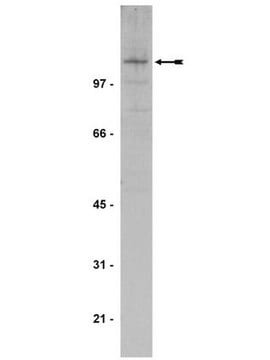
Anti-Ubiquitin, Cat. No. 07-375, is a rabbit polyclonal antibody that detects ubiquitin and ubiquitinylated proteins and is tested for use in Western Blotting.
Purified rabbit polyclonal antibody in buffer containing 0.1 M Tris-Glycine (pH 7.4), 150 mM NaCl with 0.05% sodium azide with 30% glycerol.
Specificity
This rabbit polyclonal antibody specifically detects Ubiquitin.
Immunogen
Full-length ubiquitin is isolated from bovine erythrocytes.
Application
Anti-Ubiquitin, Cat. No. 07-375, is a rabbit polyclonal antibody that detects ubiquitin and ubiquitinylated proteins and is tested for use in Western Blotting.
Research Category
Signaling
Research Sub Category
Ubiquitin & Ubiquitin Metabolism
Quality
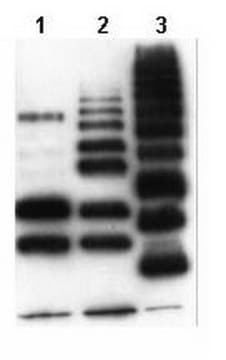
routinely evaluated by immunoblot from bovine erythrocytes, and ubiquitylated proteins in acid extracts from HeLa cells
Target description
Ubiquitin (Ub) is initially produced as a 229 amino acids Polyubiquitin-B (UniProt: P0CG47) precursor protein encoded by the UBB gene (Gene ID: 7314) or a 685 amino acids Polyubiquitin-C precursor protein (UniProt: P0CG48) encoded by the UBC gene (Gene ID: 7316) in human. Ub exists either covalently attached to another protein, or free (unanchored). When covalently bound, it is conjugated to target proteins via an isopeptide bond either as a monomer (monoubiquitin), a polymer linked via different lysine residues of the ubiquitin (polyubiquitin chains) or a linear polymer linked via the initiator methionine (Met) of the ubiquitin (linear polyubiquitin chains). Ub is linked covalently via its carboxyl terminus (Gly76) to lysine residues in target proteins. In a given target lysine residue can be linked to one single Ub molecule (monoubiquitylated) or to a chain of Ub molecules (polyubiquitylated). In a polyUb chain, Ub molecules can be linked through one of the seven lysine residues (K6, K11, K27, K29, K33, K48, and K63). Polyubiquitin chains, when attached to a target protein, have different functions depending on the lysine residue of the ubiquitin that is linked. For example, lysine 6-linked may be involved in DNA repair; lysine 11-linked is involved in endoplasmic reticulum-associated degradation (ERAD) and in cell-cycle regulation, and lysine 29-linked is involved in lysosomal degradation. Lysine 48-linked chains mark proteins for proteasomal degradation, while lysine 63-linked chains are involved in endocytosis, DNA-damage responses, and in signaling leading to activation of the transcription factor NF- B. Ubiquitin undergoes phosphorylation at the serine 57 by a ubiquitin kinase and this phosphorylation is an important modifier of ubiquitin function, particularly in response to proteotoxic stress. This phosphorylation may also be a deciding factor whether ubiquitin is recycled or degraded during multi-vesicular body sorting on endosomes. (Ref.: Hepowit, NL., et al (2020). eLife 9; e58155; Lee, S., et al. (2017) eLife. 6; e29176).
Physical form
Format: Purified
Protein A Purified immunoglobulin in PBS, 0.1% sodium azide, and 30% glycerol.
Protein A purified
Storage and Stability
Maintain for 2 years at -20°C from date of shipment. Aliquot to avoid repeated freezing and thawing. For maximum recovery of product, centrifuge the original vial after thawing and prior to removing the cap.
Analysis Note
Control
All tissue
Other Notes
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Legal Information
UPSTATE is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.