T60402
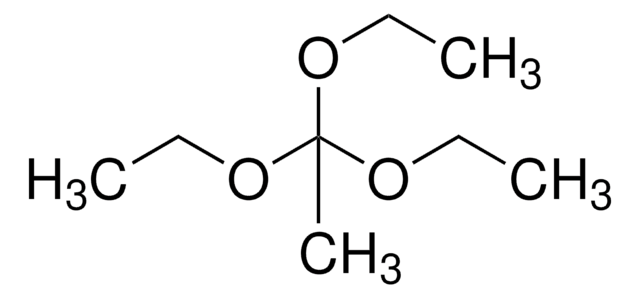
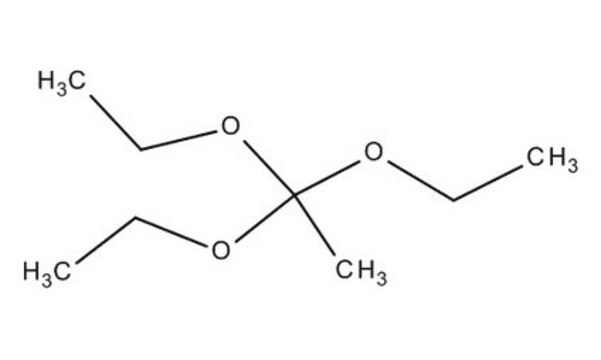
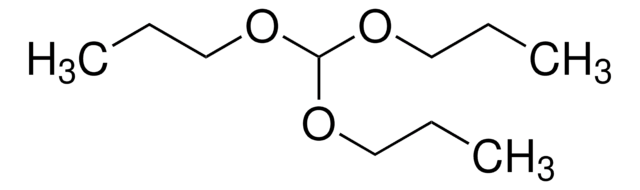
Triethyl orthoacetate
97%
Synonym(s):
1,1,1-Triethoxyethane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C(OC2H5)3
CAS Number:
Molecular Weight:
162.23
Beilstein:
506201
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.396 (lit.)
bp
142 °C (lit.)
density
0.885 g/mL at 25 °C (lit.)
SMILES string
CCOC(C)(OCC)OCC
InChI
1S/C8H18O3/c1-5-9-8(4,10-6-2)11-7-3/h5-7H2,1-4H3
InChI key
NDQXKKFRNOPRDW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Triethyl orthoacetate can be used for the synthesis of:
- Ordered mesoporous carbons for catalysis, electrochemistry and hydrogen storage applications.
- Esters of phosphonic acids, carboxylic acids and phosphinic acids.
- Ethyl alk-4-enoates by Johnson–Claisen rearrangement reaction.
- Benzoxazoles, benzimidazoles and oxazolo[4,5-b]pyridines.
- Quinazolin-4(3H)-one derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
102.2 °F - Non-equilibrium method
Flash Point(C)
39 °C - Non-equilibrium method
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Treatment of Baylis-Hillman adducts with triethyl orthoacetate in the presence of heterogeneous catalysts: a method for the stereoselective synthesis of two different types of trisubstituted alkenes
Das B, et al.
Tetrahedron Letters, 47(43), 7619-7623 (2006)
Synthesis of ordered mesoporous carbons with channel structure from an organic-organic nanocomposite
Tanaka S, et al.
Chemical Communications (Cambridge, England), 16, 2125-2127 (2005)
Silica sulfuric acid catalyzed synthesis of benzoxazoles, benzimidazoles and oxazolo [4, 5-b] pyridines under heterogeneous and solvent-free conditions
Mohammadpoor-Baltork I, et al.
Journal of the Iranian Chemical Society, 5(1), S65-S70 (2008)
A new approach to the facile synthesis of mono-and disubstituted quinazolin-4 (3H)-ones under solvent-free conditions.
Salehi P, et al.
Tetrahedron Letters, 46(41), 7051-7053 (2005)
Efficient esterification of carboxylic acids and phosphonic acids with trialkyl orthoacetate in ionic liquid
Yoshino T, et al.
Tetrahedron, 62(6), 1309-1317 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service